Indolocarbazole diboron derivative as well as preparation and application thereof
A technology of indolocarbazole and boron derivatives, which is applied in the field of indolocarbazole bis-boron derivatives, its preparation and application, and can solve the problem of unresolved color gamut, roll-off of light-emitting device efficiency, and light color coverage Narrow problems, to achieve the effect of broadening the coverage of the color gamut, increasing the speed of crossing, and improving the roll-off characteristics of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
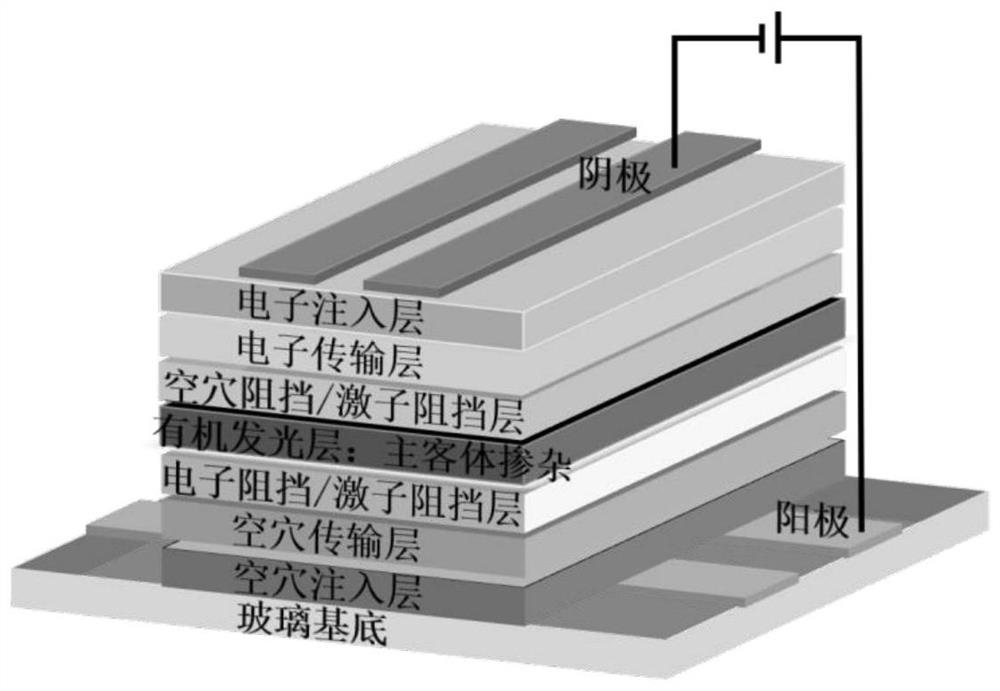
[0089] Indolecarbazole bis-boron derivatives in the present invention, its preparation method may comprise the following steps:
[0090] (1) Using aromatic amine R1 or R2 and 1-bromo-2-chloro-3-fluorobenzene as raw materials, the aromatic amine and bromine undergo Ullman reaction to obtain phenyl fluorochloride containing substituent R1 or R2.
[0091] (2) Using phenyl fluorochloride and indolocarbazole containing substituent R1 or R2 as raw materials, catalyzed by cesium carbonate, it undergoes a cross-coupling reaction to obtain indolocarbazole containing substituent R1 and R2 Chlorine derivatives of azoles.
[0092] (3) Use n-butyllithium or tert-butyllithium to carry out ortho-position metallation on the chlorine atom on the chlorine-containing derivative of indolocarbazole, and then add boron tribromide to perform lithium-boron metal exchange, A tandem bora Friedel-Crafts reaction occurs to obtain the indolocarbazole bis-boron derivative.
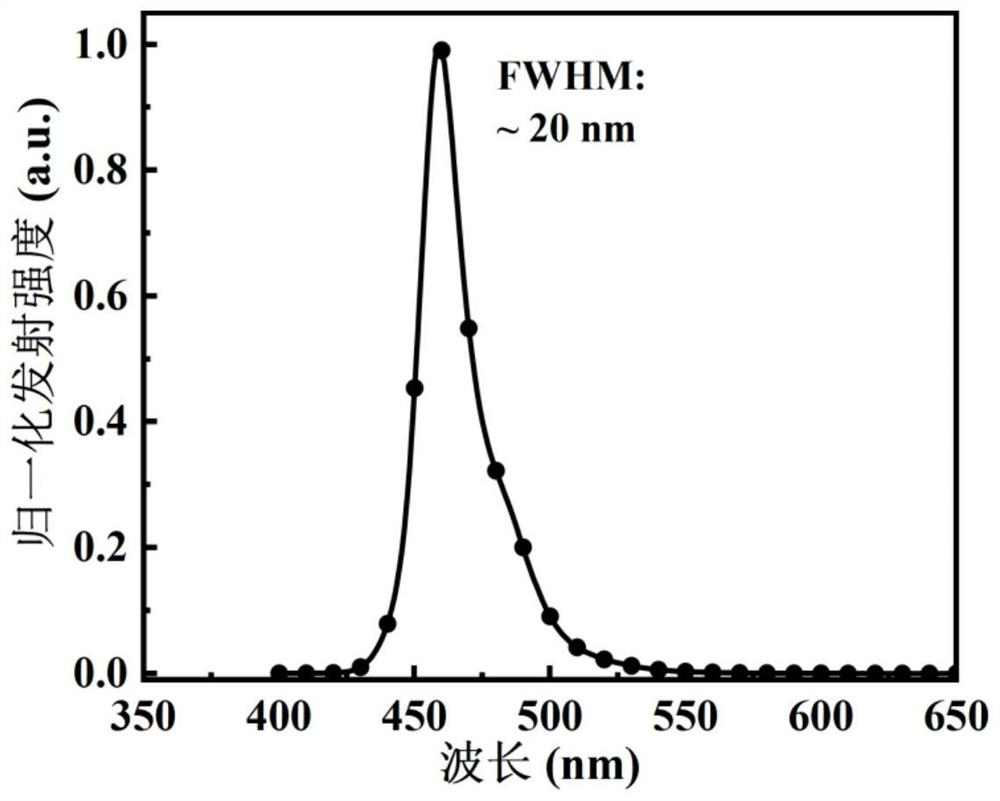
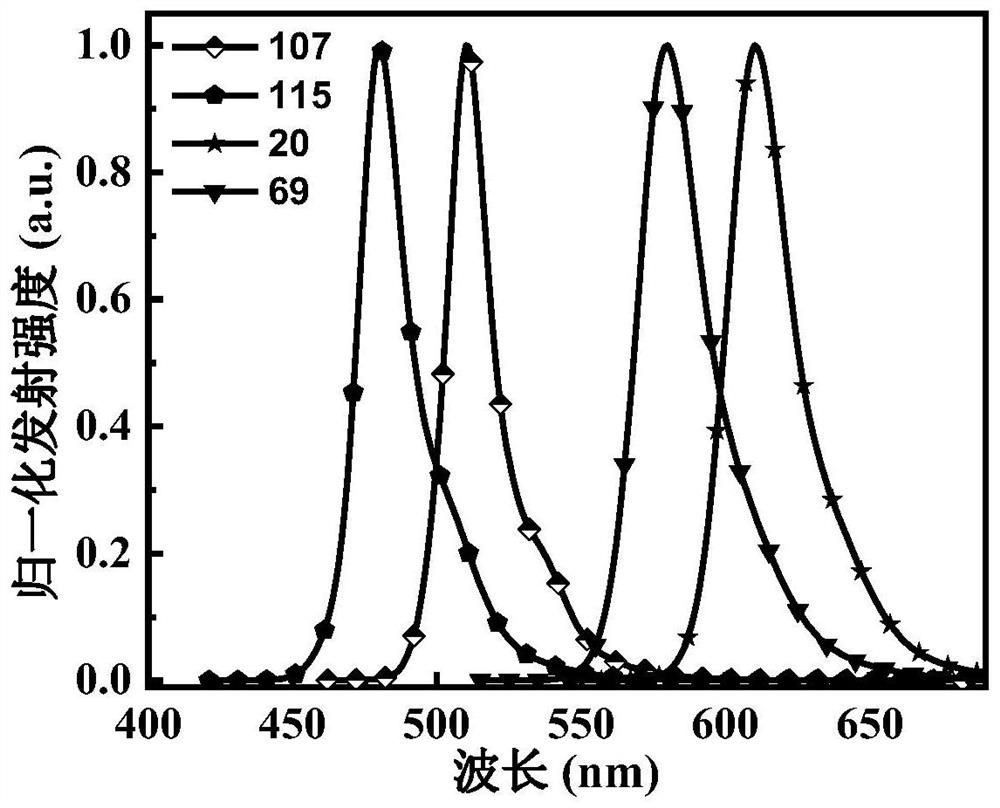
[0093] In some embodiments, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com