Method for constructing characteristic chromatograms of dried orange peel medicinal material and preparation
A characteristic map and construction method technology, applied in the field of feature map construction, can solve the problems of inability to comprehensively evaluate the quality of tangerine peel medicinal materials and their preparations, and achieve effective and reliable product quality and good separation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] In specific embodiments of the present invention, the preparation method of described need testing solution comprises:
[0074] When the substance to be tested is tangerine peel medicinal material or tangerine peel preparation in solid or semi-solid dosage form, after ultrasonic extraction of the tangerine peel medicinal material or preparation with methanol as the extraction solvent, the lost weight is supplemented by the extraction solvent, filtered, and taken Continue filtrate as need testing solution;
[0075] When the substance to be tested is a tangerine peel preparation in a liquid dosage form, the liquid can be directly taken as the test solution for analysis.
[0076] In a specific embodiment of the present invention, the preparation is a single flavor preparation of tangerine peel. Further, the single flavor preparation of orange peel is formula granules of orange peel.
[0077] In a specific embodiment of the present invention, when the substance to be test...
Embodiment 1
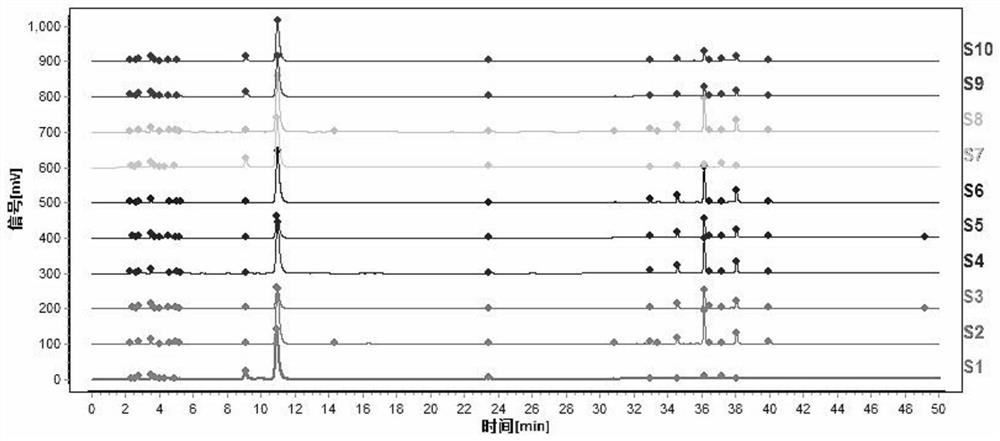
[0096] This embodiment provides a method for constructing a characteristic map of tangerine peel medicinal materials, including the following steps:
[0097] (1) Preparation of test solution
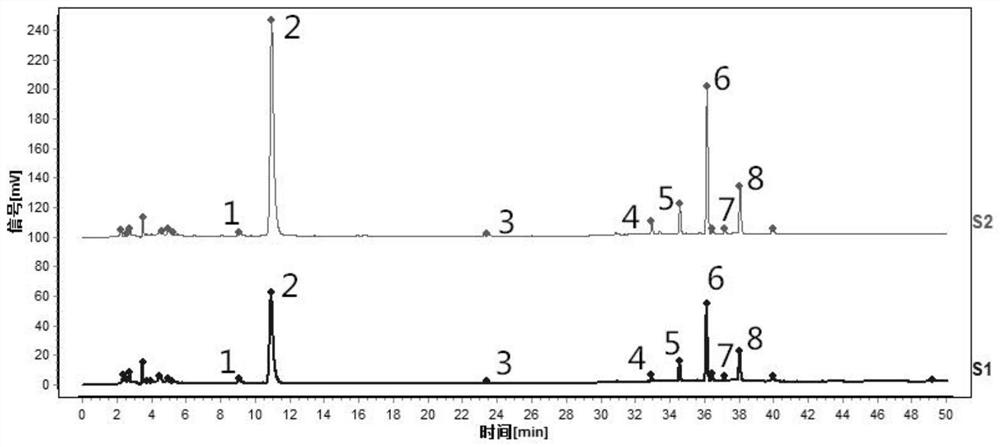
[0098] Accurately weigh 0.2g of dried tangerine peel medicinal powder (through No. 2 sieve), put it in a stoppered Erlenmeyer flask, accurately add 25mL of methanol, seal it tightly, weigh it, ultrasonically treat it (power 300W, frequency 40kHz) for 45min, let it cool, Weigh again, make up the lost weight with methanol, shake well, filter, and take the filtrate to obtain the test solution.
[0099] (2) Preparation of reference substance solution
[0100] Accurately weigh the appropriate amount of naringel rutin reference substance, hesperidin reference substance, nobiletin reference substance and tangeretin reference substance, add methanol to make a mixed solution containing 100 μg of the reference substance per 1 mL, and obtain the reference substance solution.
[0101] (3) Detectio...
Embodiment 2
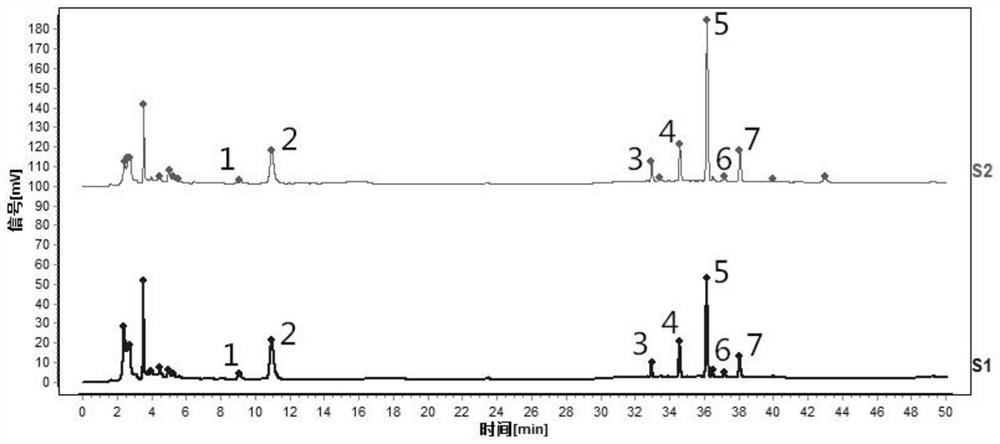
[0108] This example provides a method for constructing a characteristic map of orange peel formula granules, specifically refer to the method in Example 1, the only difference is that the preparation method of the test solution in step (1) is different.
[0109] The preparation method of the need testing solution of the present embodiment comprises:
[0110] Take an appropriate amount of tangerine peel formula granules, grind finely, accurately weigh 0.2g, place in a stoppered Erlenmeyer flask, accurately add 50mL of methanol, weigh, ultrasonically treat (power 300W, frequency 40kHz) for 30min, let cool, and weigh again , Use methanol to make up the lost weight, shake well, filter, take the continued filtrate, that is, the test solution.
[0111] Such as figure 2 Shown is the characteristic spectrum of tangerine peel formula granules (two batches). Among them, 1 # The peak corresponds to naringerin rutin, 2 # The peak is the reference peak, corresponding to hesperidin, 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com