Method and device for generating methodology verification report of clinical chromatography-mass spectrometry technology
A methodology and mass spectrometry technology, applied in the field of medical testing, can solve the problems of different data file types, typesetting, inconvenient report final review, time-consuming and labor-intensive, etc., to avoid data sorting process, save labor costs, and improve work efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
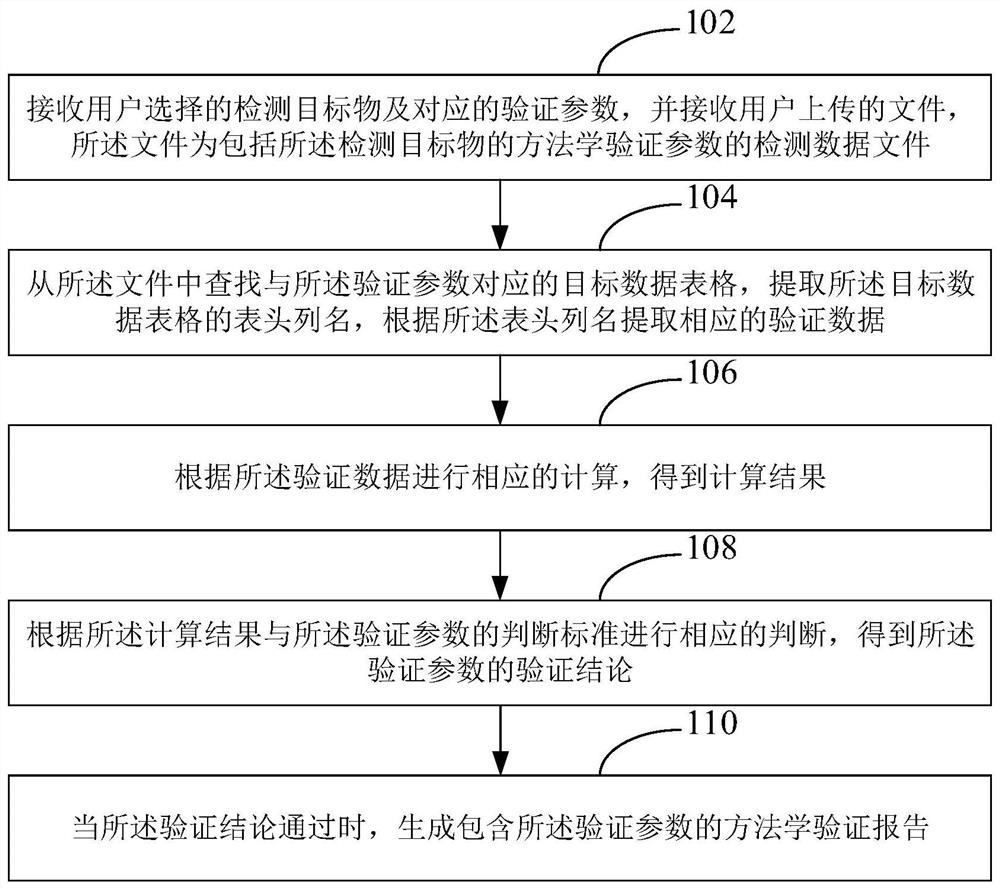
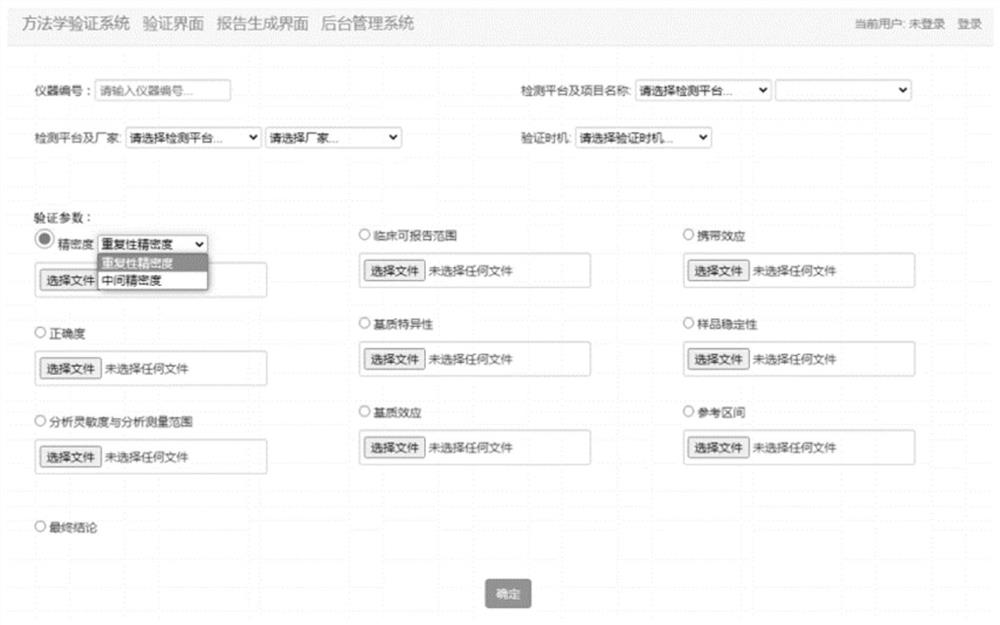
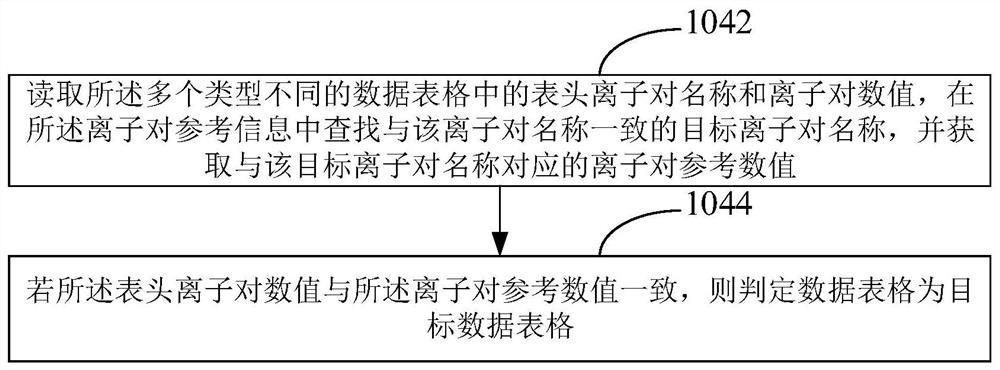
[0041] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0042] In one embodiment, chromatography has effective separation and resolution capabilities for organic compounds, while mass spectrometry is an effective means for accurately identifying compounds. The chromatographic mass spectrometry technology composed of the combination of the two can directly use chromatography to separate complex mixture samples under the control of a computer, so that the compounds in it can enter the ion source of the mass spectrometer one by one, and electron bombardment or chemical ionization can be used. All compounds in each sample are ionized. In the field of medic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com