Preparation method of lansoprazole
A technology for lansoprazole and lansoprazole crude products, which is applied in the field of medicine, can solve the problems of high production cost and low yield of lansoprazole, and achieves improved total yield, low solvent toxicity and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
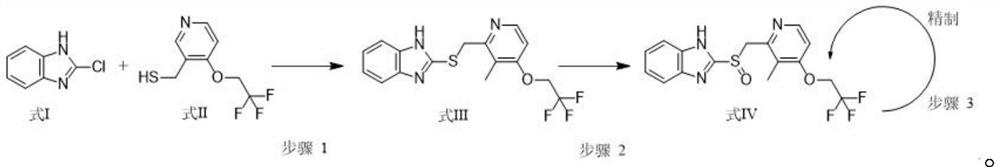
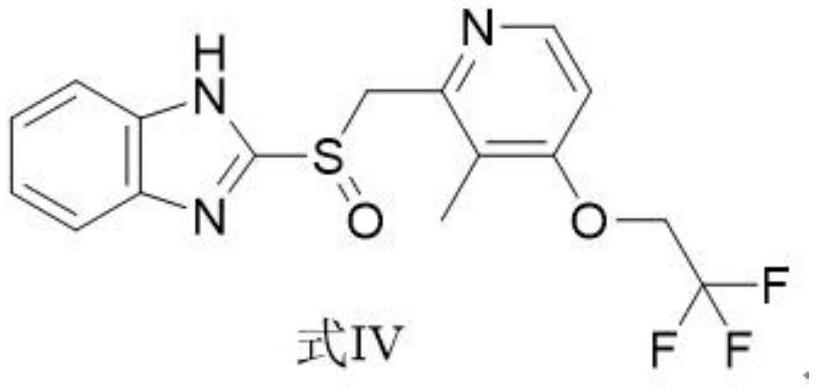
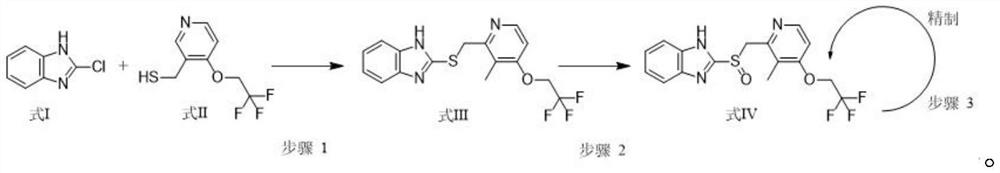
[0033] The synthetic method of 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylthio-1H-benzimidazole with structural formula (III): to 1000mL Add 400mL of ethanol to the reaction flask, then add 22.4g (100mmol) of 3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methanthiol and sodium hydroxide 0.6 g (15mmol), stirred at 40°C, added dropwise a mixed solution of 16.7g 2-chlorobenzimidazole (110mmol) and ethanol (100ml) through a constant-pressure low-liquid funnel, added Bikong and reacted at 40°C for 6h, monitored by HPLC, and the reaction After completion, 0.1 mol / L hydrochloric acid was added dropwise to the reaction bottle, the temperature was controlled at 20°C during the process, the pH was adjusted to 7, and the crystallization was stirred at 20°C for 6h. Suction filter the above system through a Buchner funnel to obtain a filter cake, rinse the solid with 44.8 g of purified water to obtain a wet product. Drying: Blast drying at 55°C for 8 hours to obtain 30.7 g (8...
Embodiment 2
[0038]The synthetic method of 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylthio-1H-benzimidazole with structural formula (III): to 1000mL Add 400mL of ethanol to the reaction flask, then add 22.4g (100mmol) of 3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methanthiol and potassium hydroxide 0.8 g (14mmol), stirred at 30°C, added dropwise a mixed solution of 19.8g 2-chlorobenzimidazole (130mmol) and ethanol (100ml) through a constant-pressure low-liquid funnel, added to complete the reaction at 30°C for 3h, monitored by HPLC, and the reaction After completion, 0.1 mol / L hydrochloric acid was added dropwise to the reaction bottle, the temperature was controlled at 10°C during the process, the pH was adjusted to 6, and the temperature was controlled at 10°C to stir and crystallize for 3h after the addition. Suction filter the above system through a Buchner funnel to obtain a filter cake, rinse the solid with 67.2 g of purified water to obtain a wet product. Drying: co...
Embodiment 3
[0043] The synthetic method of 2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine]methylthio-1H-benzimidazole with structural formula (III): to 1000mL Add 400mL of ethanol to the reaction flask, then add 22.4g (100mmol) of 3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methanthiol and 0.8 g (20mmol), stirred at 35°C, added dropwise a mixed solution of 22.8g 2-chlorobenzimidazole (150mmol) and ethanol (100ml) through a constant pressure low liquid funnel, reacted at 35°C for 5h, monitored by HPLC, and the reaction was complete Add 0.1mol / L hydrochloric acid dropwise to the reaction flask, control the temperature at 15°C during the process, adjust the pH to 6, and stir and crystallize at 15°C for 5h. Suction filter the above system through a Buchner funnel to obtain a filter cake, rinse the solid with 67.2 g of purified water to obtain a wet product. Drying: controlled temperature at 50°C and blown air for 8 hours to obtain 30.4 g (86 mmol) of the product formula III with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com