Tungsten iminoalkylene o-bit and o-binol complexes and their use in olefin metathesis reactions
An alkyl and compound technology, which can be used in metathesis reaction to produce hydrocarbons, preparation of organic compounds, catalysts of organic compounds/hydrides/coordination complexes, etc., and can solve the problems of limited knowledge of structure-activity relationship, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] Embodiment 1 (comparative example):
[0331] wxya Cl (Me 2 Pyrr)((R)-Br-TBSOBitetO)(CH(Me) 2 Synthesis of Ph) (compound 4):
[0332]
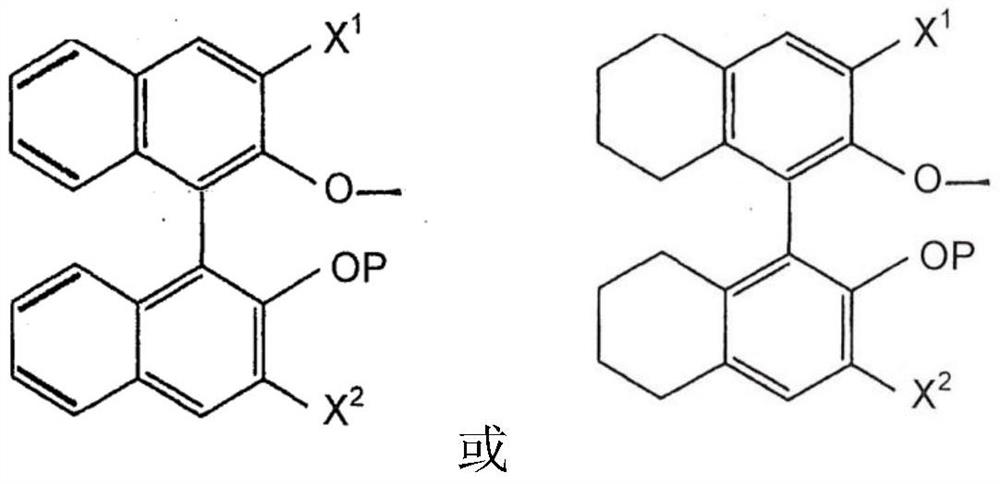
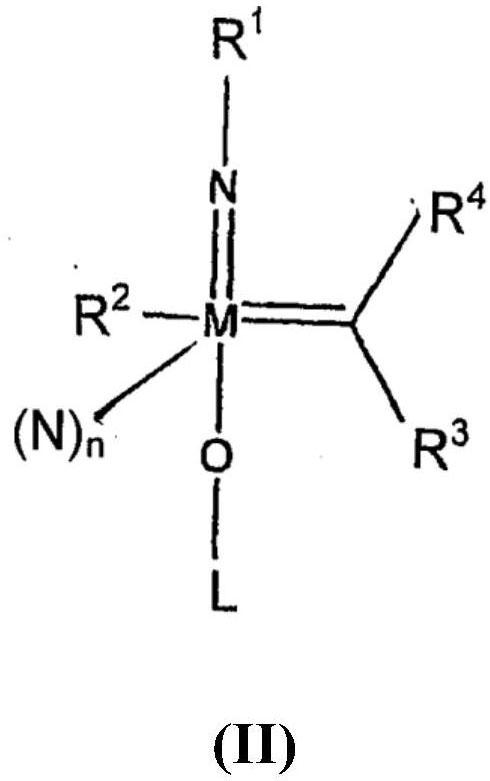
[0333] Bispyrrole precursor (WNAr Cl (Me 2 Pyrr) 2 (CHCMe 2 Ph)) and (R)-3,3'-substituted-2'-(tert-butyldimethylsilyloxy)-5,5',6,6',7,7',8,8 '-Octahydro-1,1'-binaphth-2-ol to prepare a stock solution (c=0.1M, using benzene-d6 as solvent). 100 μl of stock solutions were mixed and stirred overnight at room temperature. Then add 500 μl Benzene-d6 and pass 1 H NMR300MHz measures the sample. This solution was used in the catalytic reaction without further conversion.
[0334] Major diastereoisomer, 1H-NMR (C 6 D. 6 reference 1 H solvent=7.16ppm): -0.06(s,3H),0.11(s,3H),0.93(s,3H),1.25-1.60(m br,8H),1.69(s,H),1.73(s, H),2.26(br,6H),2.00-2.60(m,4H),5.97(br,2H),6.23(t,1H,3JHH=8.1Hz),6.85(d,2H,3JHH=8.1Hz), 6.93(m,1H),7.09(m,2H),7.16(s,1H),7.24(s,1H),7.42(m,2H),9.73(s,1H,1JCH_syn=117.8Hz, 2JWH=16.0Hz )ppm.
Embodiment 2
[0336] wxya Cl (CHCMe 2 Ph)(Me 2 Synthesis of Pyr)((R)-Br-TBSBitet-O))(MeCN) (Compound 1):
[0337]
[0338]
[0339] The reaction is filled with N 2 carried out in the glove box. The round bottom flask was equipped with a magnetic stir bar. Add initial W(NArCl)(CHCMe 2 Ph)(2,5-Me 2 Pyr) 2 The complex (0.20 g, 0.30 mmol) was then mixed with toluene (6 mL) to give a tan homogeneous solution. The ligand (R)-3,3'-substituted-2'-(tert-butyldimethylsilyloxy)-5,5',6,6',7,7' was then ,8,8'-octahydro-1,1'-binaphth-2-ol, 0.17 g, 0.30 mmol) was added to the solution as a solid. The reaction mixture was stirred overnight; the progress of the reaction was monitored by NMR. Solvent was removed under reduced pressure. The residue was dissolved in n-pentane (3 mL) to obtain an orange-red homogeneous solution. To this solution was added MeCN (18.5 mg, 24 μL, 0.45 mmol) at room temperature. After adding MeCN, a pale yellow precipitate came out of solution. Place the mixture...
Embodiment 3
[0342] W(NAr Cl ) (CHCMe 2 Ph)(Me 2 Synthesis of Pyr)((R)-Br-TBSOBitet-O)(Py)(Compound 3):
[0343]
[0344] The reaction is filled with N 2 carried out in the glove box. The round bottom flask was equipped with a magnetic stir bar. Add initial W(NAr Cl ) (CHCMe 2 Ph)(Me 2 Pyr) 2The complex (0.20 g, 0.30 mmol) was then mixed with toluene (6 mL) to give a tan homogeneous solution. Ligand (R)3,3'-substituted-2'-(tert-butyldimethylsilyloxy)-5,5',6,6',7,7', 8,8'-Octahydro-1,1'-binaphth-2-ol, 0.17 g, 0.30 mmol) was added to this solution as a solid. The reaction mixture was stirred overnight; the progress of the reaction was monitored by NMR. Solvent was removed under reduced pressure. The residue was dissolved in n-pentane (3 mL) to obtain an orange-red homogeneous solution. To this solution was added a few drops of pyridine at room temperature. After adding pyridine, a pale yellow precipitate came out of solution. Place the mixture in a glove box refrigerator fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com