Method for compounding epoxy fatty acid ester with unsaturated fatty acid ester
An epoxy fatty acid ester, fatty acid ester technology, applied in chemical recovery, organic chemistry and other directions, can solve problems such as cumbersome steps, and achieve the effects of reducing pollution, reducing production costs and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
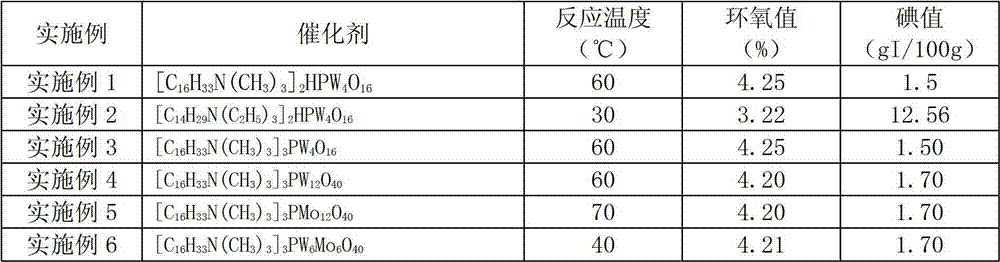
[0041] Add 3 parts by mass of catalyst (catalyst The structure is shown in Table 1), the temperature is raised to 60 degrees, and 44 mass parts of 30 mass % hydrogen peroxide aqueous solution is added dropwise. After 1 hour of reaction, the catalyst is separated out from the reaction system, and the catalyst is separated by centrifugation. The filtrate is epoxy fatty acid methyl ester and The mixture of aqueous solution, liquid separation removes the water phase to obtain an organic phase, and the organic phase decompresses to remove water to obtain a light yellow product with an iodine value of 1.5gI / 100g, an epoxy value of 4.25%, an acid value of 0.8mgKOH / g, and a water content of 0.4%. The recovered catalyst is directly added to the reaction system of the next batch, and is recycled 5 times. The iodine value of the obtained product is all less than 2gI / 100g, and the epoxy value is greater than 4.2%.
Embodiment 2-45
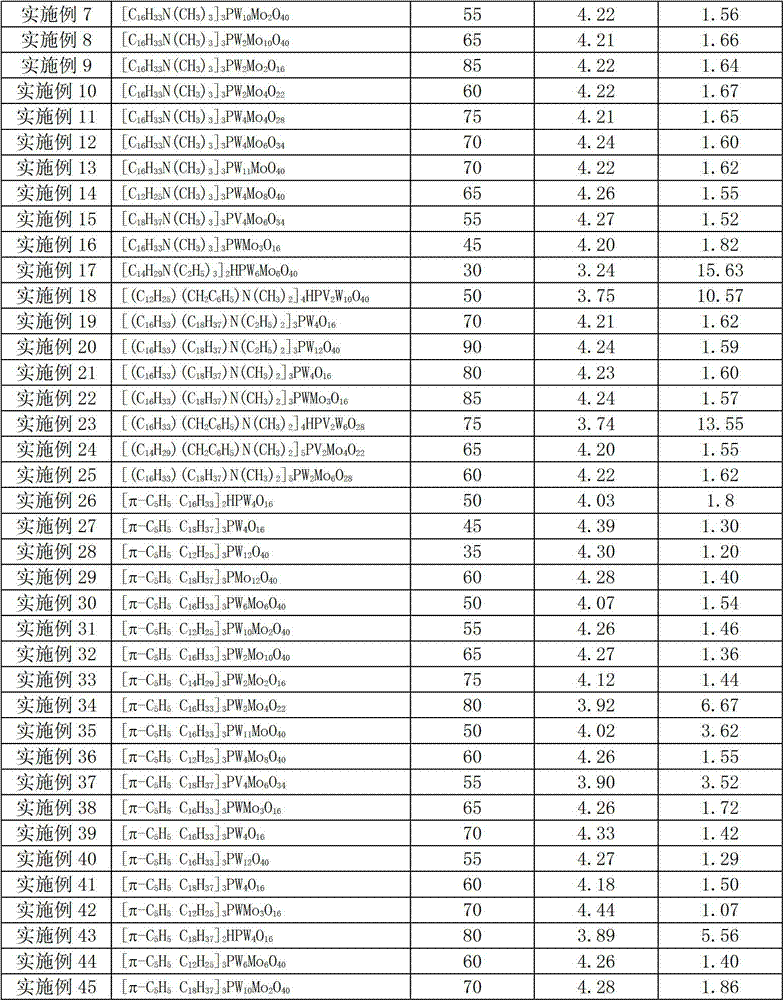
[0043] Except replacing catalyst and / or changing reaction temperature according to Table 1, carry out the epoxidation reaction of unsaturated fatty acid methyl ester according to the experimental method of embodiment 1, experimental result is shown in Table 1. After the epoxidation reaction, the catalyst is precipitated from the reaction system and centrifuged to be directly used in the next batch of reaction system for recycling.
[0044] Table 1
[0045]
[0046]
Embodiment 46
[0048] Add 3 parts by mass of catalyst [C16H 33 N(CH 3 ) 3 ] 2 HPW 4 o 16 , be warming up to 60 degree, drip 44 mass parts of aqueous hydrogen peroxide solution of 30 mass %, react after 1 hour, catalyst is separated out from reaction system, centrifugation goes out catalyst, and filtrate is the mixture of epoxy fatty acid ethyl ester and aqueous solution, separates The aqueous phase was removed from the liquid to obtain an organic phase, and the organic phase was distilled under reduced pressure to remove water to obtain a light yellow product with an iodine value of 1.4gI / 100g, an epoxy value of 4.23%, an acid value of 0.7mgKOH / g, and a water content of 0.4%. The recovered catalyst is directly added to the reaction system of the next batch, and is recycled 5 times. The iodine value of the obtained product is all less than 2gI / 100g, and the epoxy value is greater than 4.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com