Application of YM-155 in medicine for preventing and treating chronic kidney disease
A YM-155 technology for chronic kidney disease, applied in the field of medicine, can solve the problems of lack of intervention methods, achieve the effect of improving chronic kidney disease, inhibiting the transdifferentiation of renal tubular epithelial cells, and inhibiting the activation of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
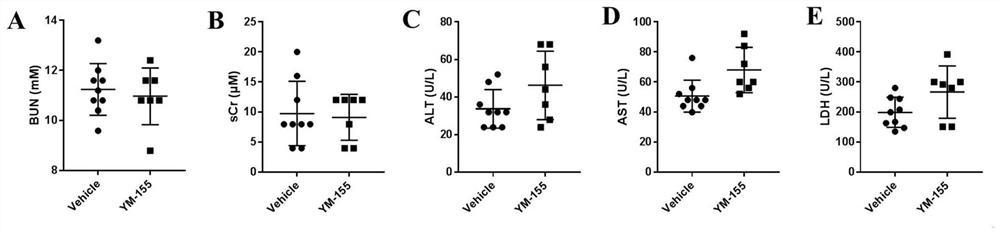
[0021] Prove that the therapeutic dose of YM-155 (3mg / kg) has no toxic side effects on normal mice:
[0022] 1. Experimental materials and methods:
[0023] The C57BL / 6 species male mice used in this example (7 weeks old at the time of purchase, weighing 20-24 g) were purchased from the Experimental Animal Center of Nanjing Medical University, and were raised in an SPF-level barrier environment in the Experimental Animal Center of Nanjing Medical University. Feed, maintain a 12h / 12h light / dark rhythm. Laboratory temperature: 20-25°C, humidity 50±5%. The mice were randomly divided into control group and YM-155 group after 1 week of adaptive feeding.
[0024] The YM-155 used in this example was purchased from Selleck Company. The dosage of the mice was 3mg / kg, and the administration method was intraperitoneal injection; Administration for 15 days. The whole blood of the mice was collected 2 hours after the last administration, and the serum was obtained by centrifugation at ...
Embodiment 2
[0029] A therapeutic dose of YM-155 (3mg / kg) improves the levels of inflammatory factors in the kidney tissue of the mouse UUO model:
[0030] 1. Experimental materials and methods:
[0031] 1.1. Establishment of unilateral ureteral obstruction (UUO) model and drug treatment:
[0032] Forty male C57BL / 6 mice (7 weeks old at the time of purchase, weighing 20-24 g) were bred in an SPF-grade barrier environment in the Experimental Animal Center of Nanjing Medical University. After adaptive feeding for 1 week, the mice were randomly divided into Vehicle+Sham group, Vehicle+UUO group, YM-155+Sham group, and YM155+UUO group.
[0033] After grouping, the mice in each group received two intraperitoneal injections of vehicle or YM-155 (3mg / kg) 24h and 2h before the UUO model respectively. Afterwards, unilateral ureteral ligation causes obstruction of the renal drainage system on the obstructed side, induces chronic renal structural damage, and simulates clinical renal interstitial fi...
Embodiment 3
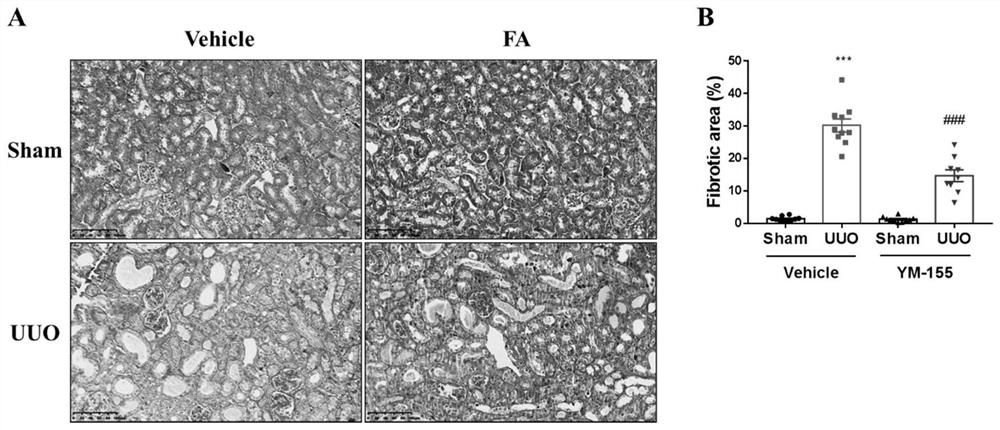
[0043] Therapeutic doses of YM-155 (3mg / kg) ameliorate renal pathological damage in the mouse UUO model:
[0044] 1. Experimental materials and methods:
[0045] Vehicle+Sham group, Vehicle+UUO group, YM-155+Sham group, YM-155+UUO group mouse modeling and administration methods were the same as in Example 2. After the last administration, the mice were sacrificed, and the left kidney tissue of Sham mice and the affected kidney tissue of UUO mice were taken, fixed with 4% paraformaldehyde at room temperature for 24 hours, dehydrated, embedded in paraffin and sectioned for Masson staining. Masson staining of kidney tissue was performed using the Masson trichrome staining solution kit from Servicebio. Use Image-ProPlus (Version 6.0.0.260) software to statistically analyze the area of Masson-stained collagen fibers. Data are represented using mean ± SEM. Analysis of variance (ANOVA) was used for comparison between multiple groups, and T test was used for data comparison betwe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com