Synthesis method of 1, 2, 3-triazole quinoxalinone derivative
A technology of triazole quinoxalinone and synthesis method, which is applied in the direction of organic chemistry and the like, can solve the problems of many steps, narrow range of substrate universality, low yield and the like, and achieves the effect of strong biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
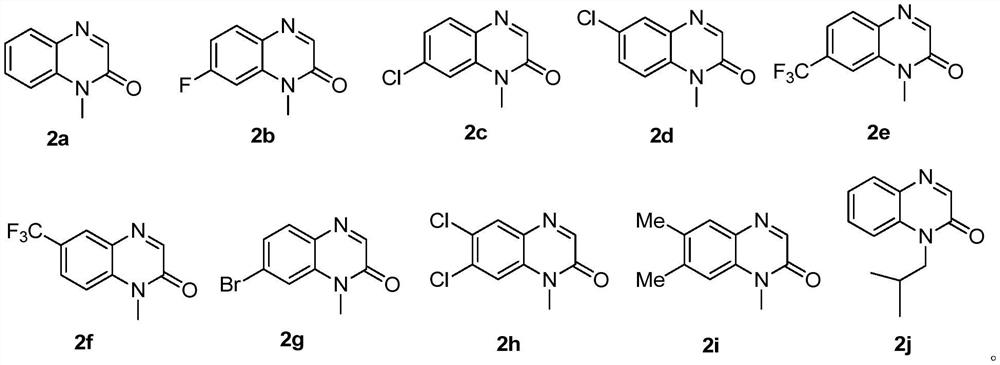
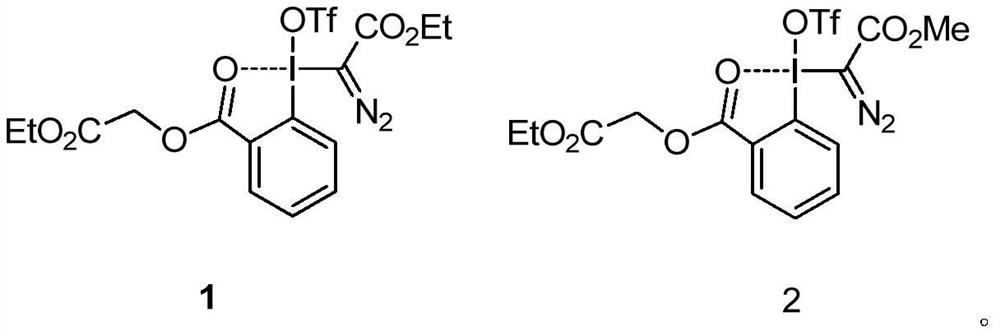
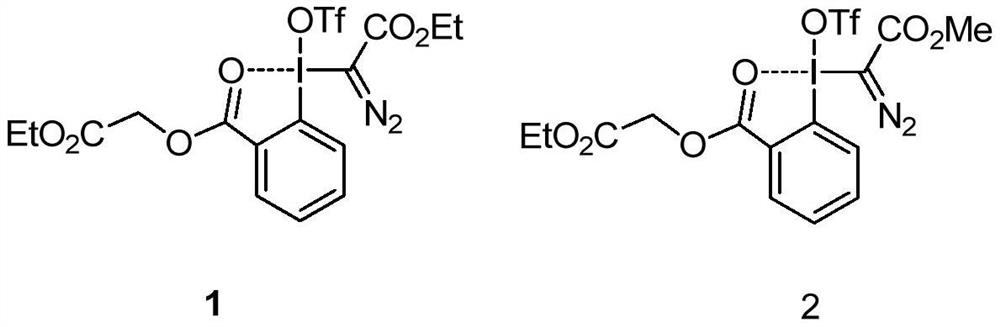
[0022] Under blue light irradiation, 1-methylquinoxalinone (0.5mmol), diazotrivalent iodine reagent 1 (0.75mmol), three (2,2'-bipyridyl) ruthenium dichloride (5%mmol) and 1,1 dichloroethane (2 mL) was added into a 15 mL test tube, reacted at room temperature for 10 hours, and separated by silica gel column chromatography to obtain the target compound 3a in a yield of 66%. 1 H NMR (300MHz, CDCl 3 )δ8.61(dd, J=8.7,1.7Hz,1H),7.68-7.62(m,1H),7.49-7.44(m,2H),4.56(q,J=7.1Hz,2H),3.77(s ,3H),1.49(t,J=7.1Hz,3H).
Embodiment 2
[0024] Under blue light irradiation, 7-fluoro-1-methylquinoxalinone (0.5mmol), diazotrivalent iodine reagent 1 (0.75mmol), tris(2,2'-bipyridyl)ruthenium dichloride (5 % mmol) and 1,1-dichloroethane (2 mL) were added to a 15 mL test tube, reacted at room temperature for 12 hours, and separated by silica gel column chromatography to obtain the target compound 3b with a yield of 75%. 1 H NMR (500MHz, CDCl 3 )δ8.33(dd,J=7.9,2.8Hz,1H),7.49-7.46(m,1H),7.42-7.40(m,1H),4.57(q,J=7.1Hz,2H),3.78(s ,3H).
Embodiment 3
[0026] Under blue light irradiation, 7-chloro-1-methylquinoxalinone (0.5mmol), diazo trivalent iodine reagent 1 (0.75mmol), three (2,2'-bipyridyl) ruthenium dichloride (5 % mmol) and 1,1-dichloroethane (2 mL) were added to a 15 mL test tube, reacted at room temperature for 12 hours, and separated by silica gel column chromatography to obtain the target compound 3c with a yield of 80%. 1 H NMR (300MHz, CDCl 3 )δ8.53(d, J=2.0Hz, 1H), 7.53(dd, J=8.9, 2.2Hz, 1H), 7.34(d, J=9.0Hz, 1H), 4.48(q, J=7.1Hz, 2H), 3.67(s, 3H), 1.41(t, J=7.1Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com