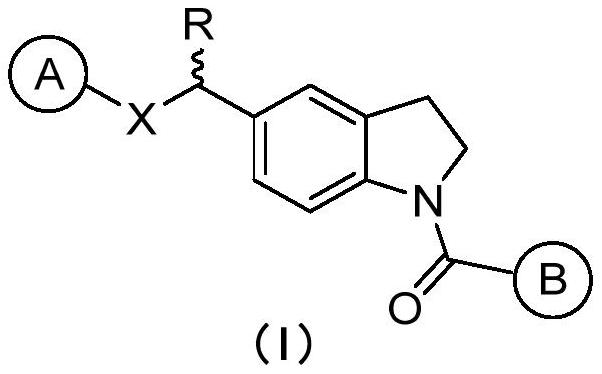
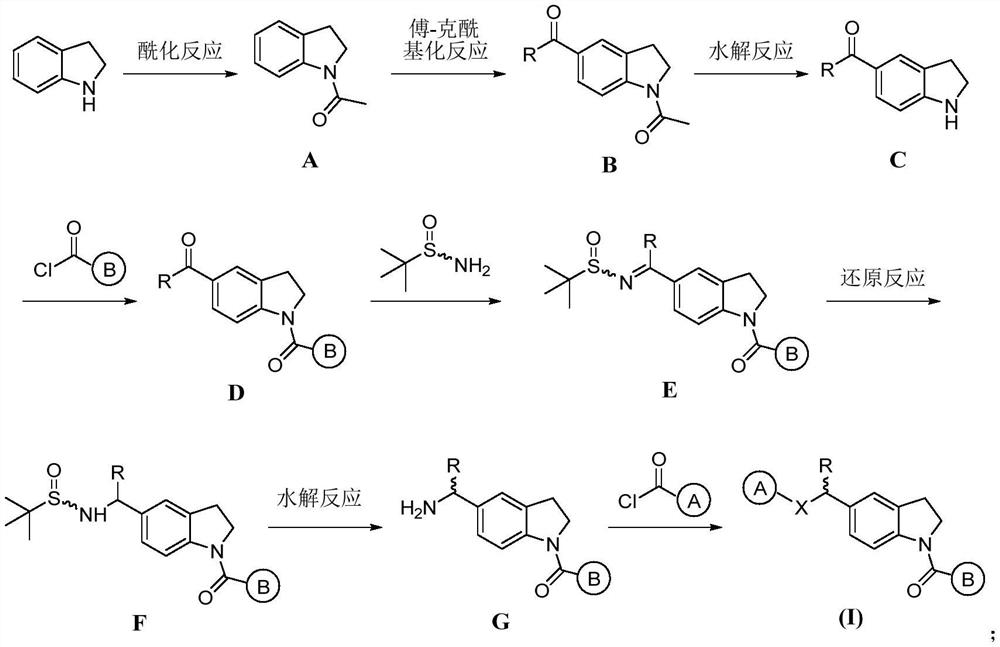
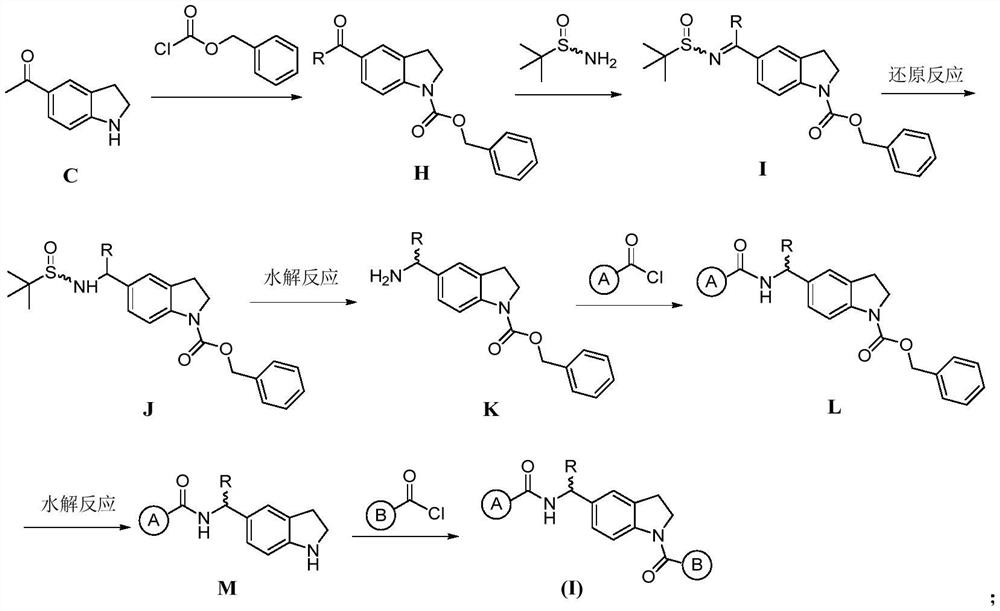
Indoline compound as well as derivative, preparation method, pharmaceutical composition and application thereof
A compound, indoline technology, applied in the field of indoline compounds and their derivatives, can solve the problems of low activity and insufficient druggability, and achieve the effects of simple preparation method, excellent curative effect and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: (R)-N-(1-(1-(cyclohexanecarbonyl)-2,3-dihydro-1H-indol-5-yl)ethyl)-4-fluorobenzamide ( 1) synthesis
[0069]
[0070] Synthesis of N-acetylindoline (1A)
[0071] 2,3-Dihydro-1H-indole (10.00g, 83.91mmol) and triethylamine (25.47g, 251.73mmol) were dissolved in 40mL of anhydrous dichloromethane, and acetyl chloride (9.88 g, 125.87mmol) in anhydrous dichloromethane (10mL), react overnight at room temperature, concentrate under reduced pressure, add 100mL of water, extract with dichloromethane (3×80mL), combine the organic phases, wash with saturated brine, and anhydrous sulfuric acid Dry over magnesium, filter with suction, concentrate under reduced pressure, and separate and purify by column chromatography to obtain 11.78 g of white solid with a yield of 87.1%. MS(ESI)m / z:160.1[M-H] - ; 1 H NMR (300MHz, DMSO-d 6 )δ (ppm) 8.05 (d, J = 8.1Hz, 1H), 7.21 (d, J = 7.2Hz, 1H), 7.14 (t, J = 7.5Hz, 1H), 6.97 (t, J = 7.5Hz, 1H), 4.05(t, J=7.8Hz, 2H), 3.12(t,...
Embodiment 2
[0086] Embodiment 2: (R)-N-(1-(1-(cyclohexanecarbonyl)-2,3-dihydro-1H-indol-5-yl)ethyl)-4-chlorobenzamide ( 2) synthesis
[0087]
[0088] Dissolve 1G (0.080g, 0.29mmol) and triethylamine (0.088g, 0.87mmol) in 3mL of anhydrous dichloromethane, slowly add 4-chlorobenzoyl chloride (0.061g, 0.35mmol) dropwise at 0°C Anhydrous dichloromethane (1 mL) solution, reacted at room temperature for 3 h, concentrated under reduced pressure, added 30 mL of water, extracted with dichloromethane (3×20 mL), combined organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, and suction filtered. Concentrate under reduced pressure, and separate and purify by column chromatography to obtain 0.10 g (99.1% ee) of white solid with a yield of 83.3%. MS(ESI)m / z:409.2[M-H] - ; 1 H NMR (300MHz, DMSO-d 6 )δ (ppm) 8.82 (d, J = 8.1Hz, 1H), 8.01 (d, J = 8.1Hz, 1H), 7.90 (d, J = 8.4Hz, 2H), 7.54 (d, J = 8.7Hz, 2H), 7.24(s, 1H), 7.14(d, J=8.4Hz, 1H), 5.12-5.08(m, 1H), 4.14(t...
Embodiment 3
[0089] Example 3: (R)-N-(1-(1-(cyclohexanecarbonyl)-2,3-dihydro-1H-indol-5-yl)ethyl)-4-cyanobenzamide (3) synthesis
[0090]
[0091] 1G (0.13g, 0.48mmol) and triethylamine (0.14g, 1.43mmol) were dissolved in 3mL of anhydrous dichloromethane, and 4-cyanobenzoyl chloride (0.080g, 0.48mmol) was slowly added dropwise at 0°C Anhydrous dichloromethane (2mL) solution, reacted at room temperature for 3h, concentrated under reduced pressure, added 30mL of water, extracted with dichloromethane (3×20mL), combined organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, and suction filtered , concentrated under reduced pressure, and separated and purified by column chromatography to obtain 0.17 g (99.5% ee) of a white solid, with a yield of 89.5%. MS(ESI)m / z:400.2[M-H] - ; 1 H NMR (300MHz, DMSO-d 6 )δ (ppm) 9.00 (d, J = 7.8Hz, 1H), 8.04-7.95 (m, 5H), 7.25 (s, 1H), 7.15 (d, J = 8.7Hz, 1H), 5.15-5.06 (m ,1H),4.14(t,J=8.4Hz,2H),3.11(t,J=8.1Hz,2H),1.79-1.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com