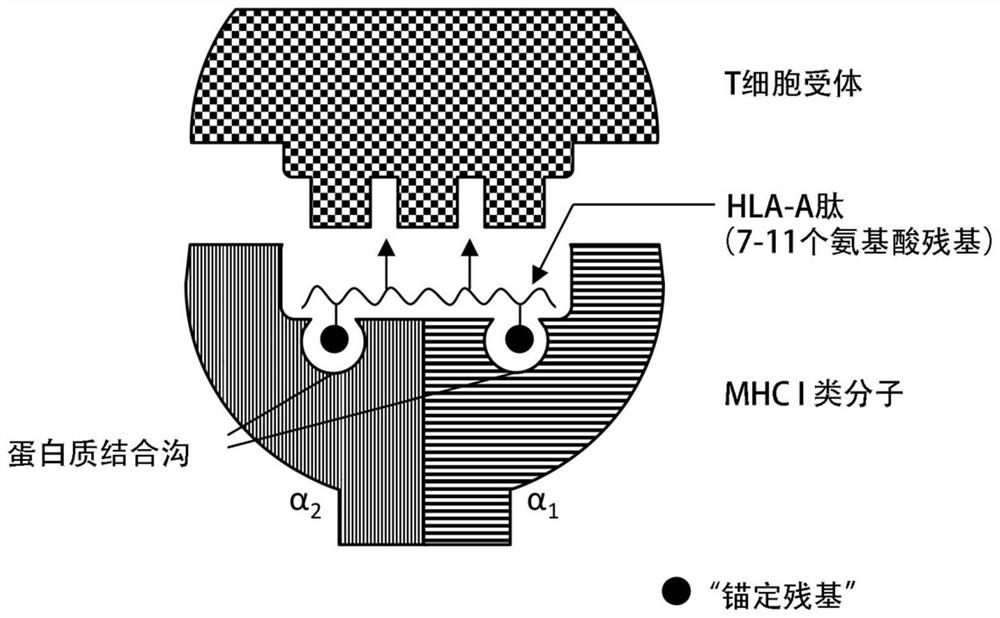
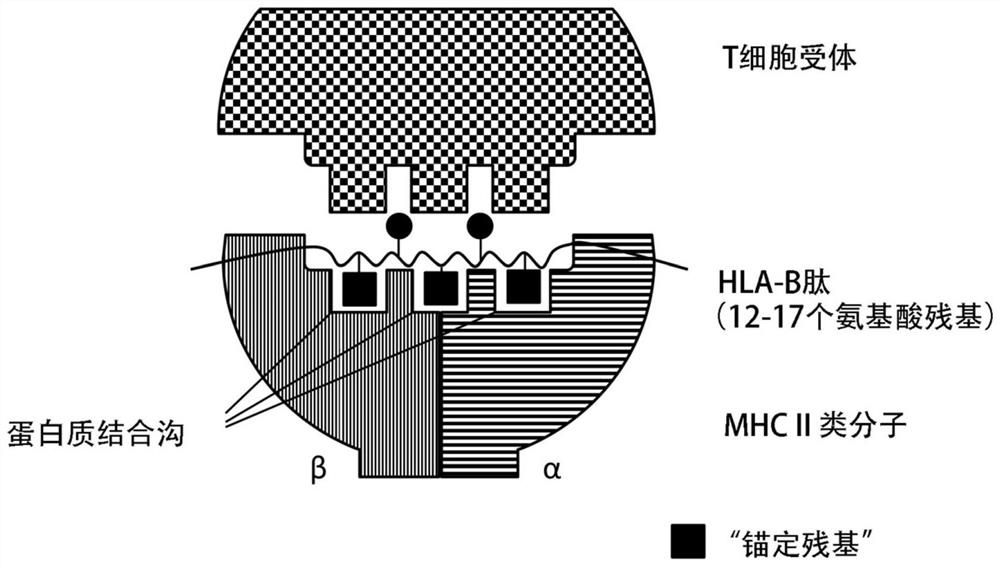
Class I and class II HLA tumor antigen peptides for treatment of breast/breast cancer
A tumor antigen peptide, breast cancer technology, applied in the direction of cancer antigen components, breast cancer vaccines, anti-tumor drugs, etc., can solve the problem of not being applicable to other patients with the same tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0361] Example 1: Transcriptome analysis [mRNA expression]
[0362] Transcriptome analysis (sequencing) was performed on all patients using the PANTHER array analysis (44K array) from Agilent Technologies. The 44K chip used contained 44,000 gene probes, so in each case, each patient could be analyzed with the chip for the activity of more than 34,000 gene expression markers. In each case, 1000 genes with a 10-fold increase in association were identified.
[0363] Assessments were based on published gene signatures known to experts.
Embodiment 2
[0364] Example 2: EXOM sequencing
[0365] Exome sequencing is performed on standard frozen specimens from patients with tumor DNA extracted. Next-generation sequencing of the exome was performed from tumor samples, covering more than 95% of the entire human coding exonic region. To this end, more than 290,000 relevant sections of DNA were selectively amplified and sequenced by semiconductor sequencing technology, among other things.
[0366] The purpose of the analysis was to identify possible mutations in order to design individual tumor antigen peptide immunizations. To exclude germline non-tumor-associated variants (SNPs, single nucleotide polymorphisms), DNA was simultaneously isolated from nucleated blood cells of the patients and sequenced using the same method for comparison. Differentiation of potential neoantigen candidates proceeds through the following six steps:
[0367] 1) Reduced selection for tumor / somatic mutations by excluding non-tumor-specific polymorphi...
Embodiment 3
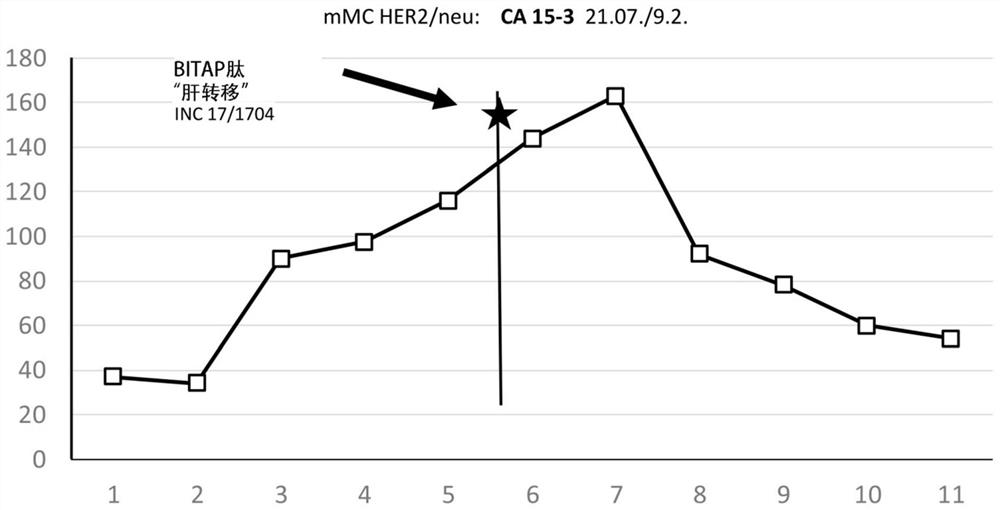
[0392] Example 3: Patient with mMC (metastatic breast cancer), type_HER2-neu
[0393] 1) The clinical history of the patient
[0394] patient, female
[0395] preliminary clinical findings : Invasive ductal bifocal breast cancer (breast CA), right side, tumor 2cm to 5cm maximum (pT2), pN1sn pN1(3 / 5)G2, NO, ER-, PR-, HER-2neu:3+, Ki-67: 55%.
[0396] Treatment procedure:
[0397] After 2 weeks: 6 cycles of neoadjuvant chemotherapy (TCH w3), trastuzumab;
[0398] ■After 6 months: irradiate the right breast + lymphatic drainage area (LAG).
[0399] The tumor was completely destroyed by radiotherapy or chemotherapy (CR=complete remission), and the result: the patient was cured and discharged.
[0400] relapse : local recurrence with additional liver metastases (4 lesions) occurred 24 months after initial diagnosis; results: ER-, PR+ (60%), HER-2neu: 3+, Ki-67: 80%.
[0401] This recurrent breast / mammary CA was accompanied by liver metastases and was significantly more ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com