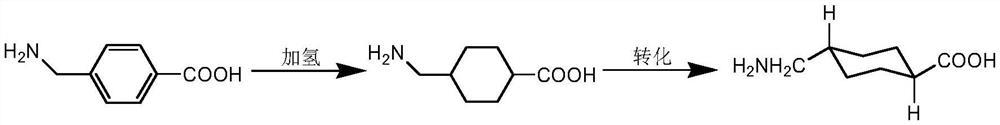
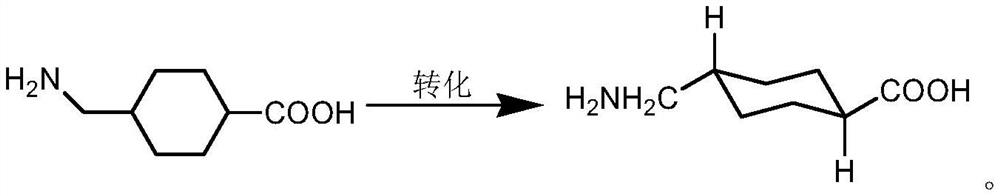
Catalyst, application and preparation method of trans-tranexamic acid
A technology of trans-tranexamic acid and tranexamic acid, which is used in the preparation of cyanide reactions, the preparation of organic compounds, organic chemical methods, etc., to reduce consumption, avoid the generation of barium sulfate solid by-products, and increase the proportion of Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] 1. The preparation process of the hydrogenation reaction solution: add 1.5L of purified water, 50g of aminotoluic acid, and 24g of concentrated sulfuric acid into a 2L pressure reaction vessel, heat up to 50-60°C to dissolve the aminotoluic acid, then add 3.5g of platinum dioxide, Airtightly start to flow hydrogen, control the temperature at 50-60°C to react for 6 hours, cool down to room temperature and filter, the filtrate is the hydrogenation reaction liquid.
[0025] Two, the purity analysis of tranexamic acid adopts the following methods:
[0026] Chromatographic column: end-capped octadecylsilane bonded silica gel column C18 0.25m×4.6mm, 5μm
[0027] Flow rate: 0.9mL / min;
[0028] Detection wavelength: 210nm;
[0029] Injection volume: 40μL;
[0030] Column temperature: 30°C;
[0031] Run time: 2.5 times the retention time of tranexamic acid;
[0032] Mobile phase: Dissolve 11g of anhydrous sodium dihydrogen phosphate or 14.3g of dihydrogen sodium phosphate i...
Embodiment 1
[0035] Embodiment 1: Concentrate the aminotoluic acid hydrogenation reaction solution to about 20% of the mass concentration of tranexamic acid, add cesium hydroxide equivalent to 0.6 equivalents of aminotoluic acid added to the reaction system, heat up to 200 ° C, and keep the temperature Pressure reaction for 15 hours, the reaction is over, the reaction solution is concentrated to about 50% of the mass concentration of tranexamic acid, the ice water is cooled to about 10 ℃ to crystallize, and tranexamic acid is obtained by filtration. The purity of tranexamic acid is 99.2% by HPLC analysis. The body content is 0.4%.
Embodiment 2
[0036] Example 2: Concentrate the hydrogenation reaction solution of aminotoluic acid to about 30% of the mass concentration of tranexamic acid, add cesium oxide equivalent to 1 equivalent of aminotoluic acid added to the reaction system, raise the temperature to 150° C., keep the temperature and maintain the pressure After reacting for 20 hours, the reaction was completed. Concentrate the reaction solution until the concentration of tranexamic acid was about 50%, cooled the ice water to about 10°C to crystallize, and filtered to obtain tranexamic acid. The purity of tranexamic acid was 99.5% according to HPLC analysis. The content is 0.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com