Preparation method of 5-methoxytryptamine
A technology of methoxytryptamine and methoxy, which is applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, great environmental hazards, unfavorable industrial production operations, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The present embodiment provides a kind of preparation method of 5-methoxytryptamine, the preparation method of described 5-methoxytryptamine comprises the following steps:
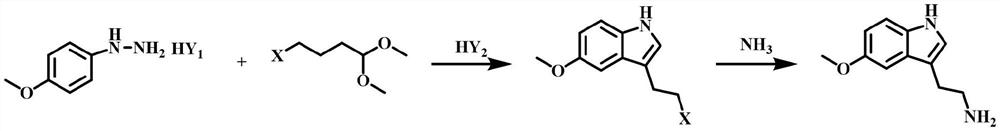
[0064] The synthetic route is:
[0065]
[0066] (1) Add 4-methoxyphenylhydrazine hydrochloride (250g, 1.43mol), ethanol (750g), 30% hydrochloric acid (75g , 0.62mol), under stirring, add 4-chlorobutyraldehyde dimethyl acetal (250g, 1.64mol) at one time, heat up to 75°C, react for 12h, TLC shows that the reaction is complete, stop heating, cool down to 55°C, concentrate under reduced pressure Obtain 600g of concentrated solution, the purity of 3-(2-haloethyl)-5-methoxyl-1H-indole in this concentrated solution is 98.2%;
[0067] (2) In a 5L autoclave with stirring and a thermometer, add the concentrated solution (300g) obtained in step (1), ethanol (900g), 27% ammonia water (480g), check that the seal is good, stir evenly, and heat up to 85°C After 12 hours of reaction, heating was stopped, the ...
Embodiment 2
[0071] The present embodiment provides a kind of preparation method of 5-methoxytryptamine, the preparation method of described 5-methoxytryptamine comprises the following steps:
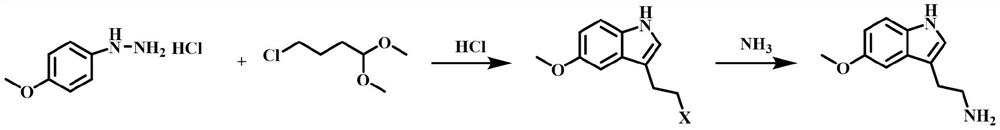
[0072] The synthetic route is:
[0073]
[0074] (1) Add 4-methoxyphenylhydrazine hydrochloride (500g, 2.86mol), ethanol (1500g), 32% hydrochloric acid (150g , 1.24mol), under stirring, add 4-chlorobutyraldehyde dimethyl acetal (500g, 3.25mol) at one time, heat up to 70°C, react for 13h, TLC shows that the reaction is complete, stop heating, cool down to 50°C, concentrate under reduced pressure Obtain 1252g of concentrated solution, the purity of 3-(2-haloethyl)-5-methoxyl-1H-indole in this concentrated solution is 98.5%;
[0075] (2) In a 5L autoclave with stirring and a thermometer, add the concentrated solution (600g) obtained in step (1), ethanol (1800g), 25% ammonia water (960g), after checking that the seal is good, stir evenly, and heat up to 80°C , after reacting for 13 hours, stop heat...
Embodiment 3
[0079] The present embodiment provides a kind of preparation method of 5-methoxytryptamine, the preparation method of described 5-methoxytryptamine comprises the following steps:
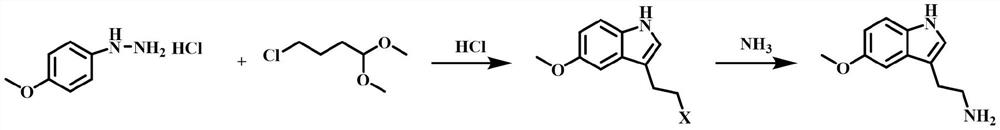
[0080] The synthetic route is:
[0081]
[0082] (1) Add 4-methoxyphenylhydrazine hydrochloride (100g, 0.57mol), ethanol (300g), 36% hydrochloric acid (30g , 0.25mol), under stirring, add 4-chlorobutyraldehyde dimethyl acetal (100g, 0.66mol) at one time, raise the temperature to 85°C, react for 10h, TLC shows that the reaction is complete, stop heating, cool down to 60°C, concentrate under reduced pressure Obtain 235g of concentrated solution, the purity of 3-(2-haloethyl)-5-methoxyl-1H-indole in this concentrated solution is 99.0%;
[0083] (2) In a 5L autoclave with stirring and a thermometer, add the concentrated solution (120g) obtained in step (1), ethanol (360g), 28% ammonia water (190g), check that the seal is good, stir evenly, and heat up to 90°C After 10 hours of reaction, heating was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com