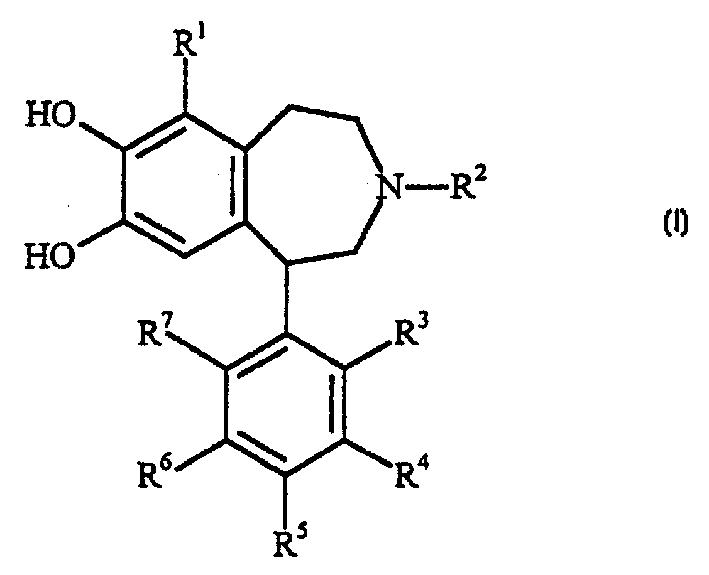
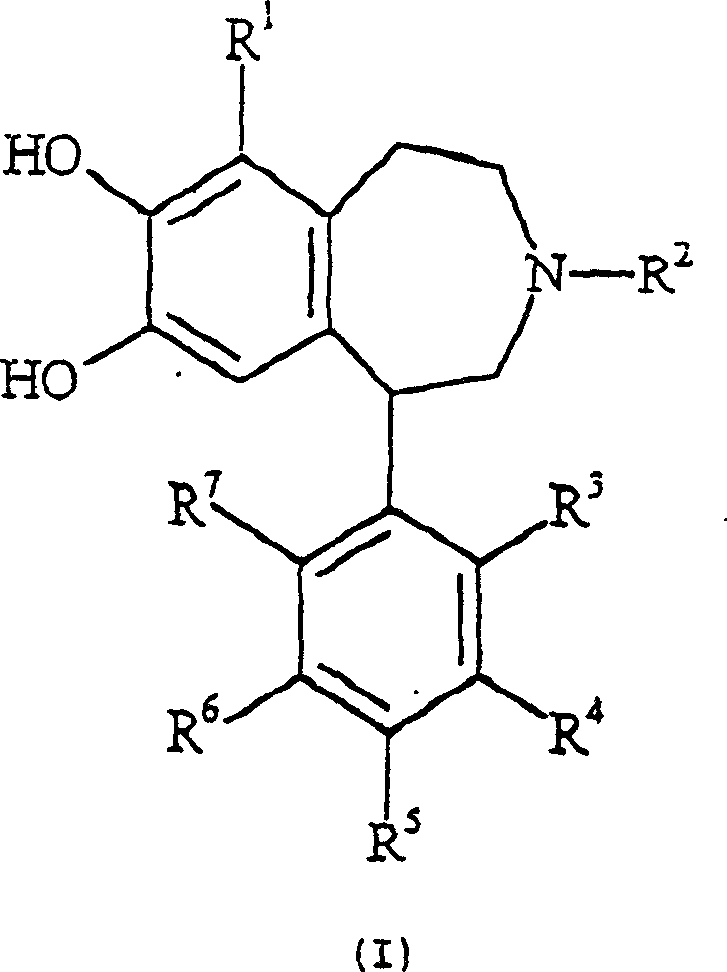
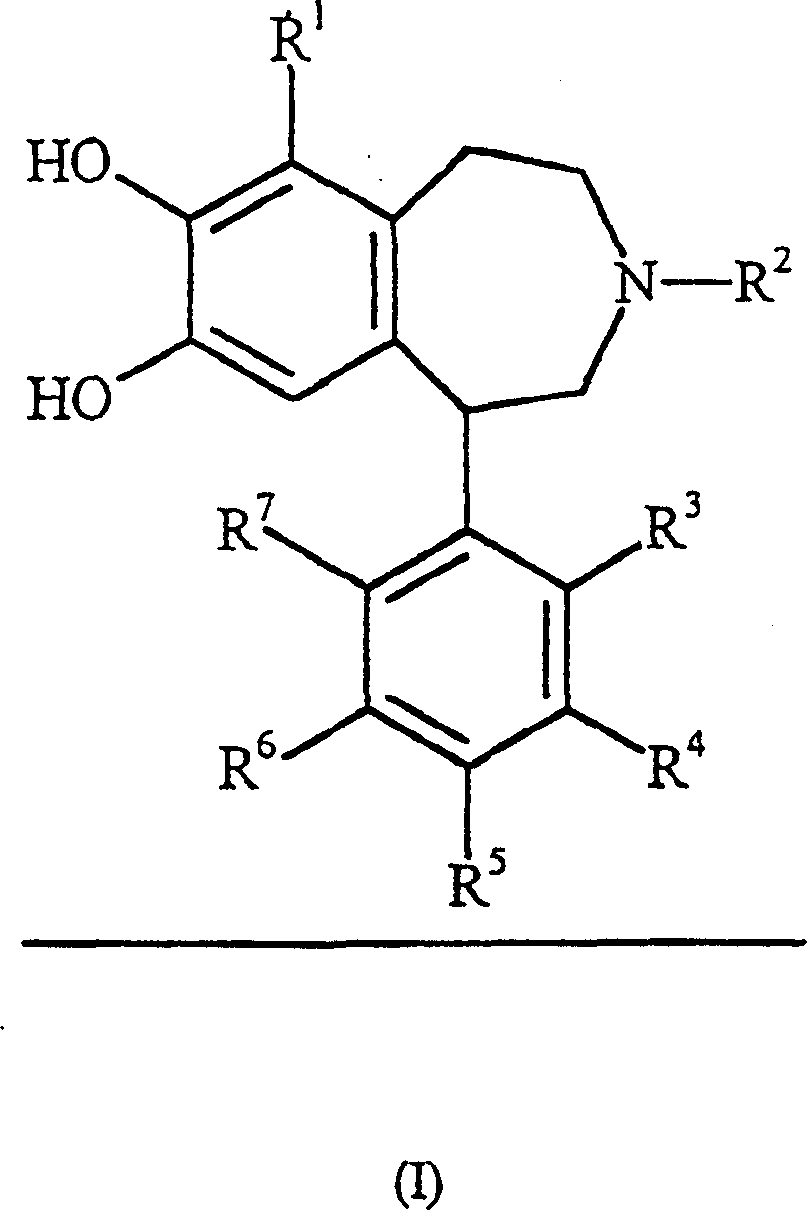
Dopamine D1 receptor agonist compounds
A receptor stimulant, dopamine technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve problems such as abnormalities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] a) 1-(benzofuran-7-yl)-2-[2-(2-chloro-3,4-dimethoxyphenyl)ethylamino]ethanol
[0025] A solution of 2-chloro-3,4-dimethoxyphenylethylamine (6.35g, 0.0296mol) and (7-benzofuryl)oxirane (4.29g, 0.0268mol) in 15ml of acetonitrile Reflux for 16 hours. The reaction mixture was cooled to 0°C (ice bath). Filtration and recrystallization of the crude product from hot acetonitrile afforded the title compound (3.52 g, 35%) as a white crystalline solid. 1 H-NMR (CDCl 3 )[δ, ppm]: 2.86-3.16 (m, 6H); 3.85 (s, 6H); 5.26 (dd, 1H); 6.73-6.77 (m, 2H); 6.91 (d, 1H); m, 1H); 7.39(d, 1H); 7.51(d, 1H); 7.61(d, 1H).
[0026] b) 1-(benzofuran-7-yl)-6-chloro-7,8-dimethoxy-2,3,4,5-tetrahydro-1H-3-benzazepine
[0027] 1-(benzofuran-7-yl)-2-[2-(2-chloro-3,4-dimethoxyphenyl) in 70ml trifluoroacetic acid was treated with concentrated sulfuric acid (0.71ml, 0.0135mol) ) ethylamino]ethanol (2.20 g, 5.85 mmol), and stirred at room temperature for 90 minutes. The solution was evaporated in vacuo...
Embodiment 2
[0033] a) 1-(Benzo[b]thiophen-7-yl)-2-[2-(2-chloro-3,4-dimethoxyphenyl)ethylamino]ethanol
[0034] A mixture of 2-chloro-3,4-dimethoxyphenylethylamine (7.00g, 32.5mmol) and 7-benzo[b]thienyloxirane (5.30g, 30.1mmol) in 20ml of acetonitrile The solution was refluxed for 72 hours. The reaction mixture was cooled to 0°C (ice bath), filtered and the crude product recrystallized from hot acetonitrile to afford the title compound (5.57 g, 47%) as a white crystalline solid. 1 H-NMR (CDCl 3 )[δ, ppm]: 2.83-3.08(m, 6H); 3.84(s, 3H); 3.85(s, 3H); 5.06(m, 1H); 6.73(d, 1H); 6.88(d, 1H) ; 7.35 (m, 3H); 7.43 (d, 1H); 7.73 (m, 1H).
[0035] b) 1-(Benzo[b]thiophen-7-yl)-6-chloro-7,8-dimethoxy-2,3,4,5-tetrahydro-1H-3-benzazepine
[0036] 1-(Benzo[b]thiophen-7-yl)-2-[2-(2-chloro-3 , 4-dimethoxyphenyl)ethylamino]ethanol (3.90 g, 10 mmol), and the solution was heated to reflux for 18 hours. The solution was evaporated in vacuo and the residue was dissolved in dichloromethane (100ml), the re...
Embodiment 3
[0041] a) 2-(2-chloro-3,4-dimethoxyphenyl)ethylamino]-1-indan-5-yl-ethanol
[0042] To a solution of 2-indan-5-yloxirane (3.38 g, 21.1 mmol) in anhydrous acetonitrile (20 ml) was added 2-(2-chloro-3,4-dimethoxyphenyl) Ethylamine (2.0 g, 23.2 mmol), and the solution was refluxed for 20 hours. Upon cooling a white precipitate formed which was collected by filtration and washed with diethyl ether to give the title compound as a white solid (2.85 g, 36%).
[0043] 1 H NMR (400 MHz, DMSO-d6): δ (ppm) 1.96-2.03 (2H, m, CH 2 -CH 2 -CH 2 ), 2.50-2.84 (10H, m, 5xCH 2 ), 3.72 (3H, s, CH 3 O), 3.80 (3H, s, CH 3 O), 4.56 (1H, t, J 6.04, H-1), 5.14 (1H, broad, NH) and 6.94-7.14 (5H, m, Ar-H). This material was used in the next reaction without further purification.
[0044] b) 1-indan-5-yl-6-chloro-7,8-dimethoxy-2,3,4,5-tetrahydro-1H-3-benzazepine
[0045] 2-[(2-Chloro-3,4-dimethoxyphenyl)ethylamino]-1-indan-5-yl-ethanol (2.7g, 7.18mmol) was dissolved in trifluoroacetic acid (50m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com