Application of PF543 in preparation of PD-L1/PD-1 monoclonal antibody tumor immunotherapy drug
A technology of PD-L1 and anti-tumor immunity, applied in the field of biological, PD-L1/PD-1 monoclonal antibody tumor immunotherapy, can solve the problems of complex tumor microenvironment and sexual drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
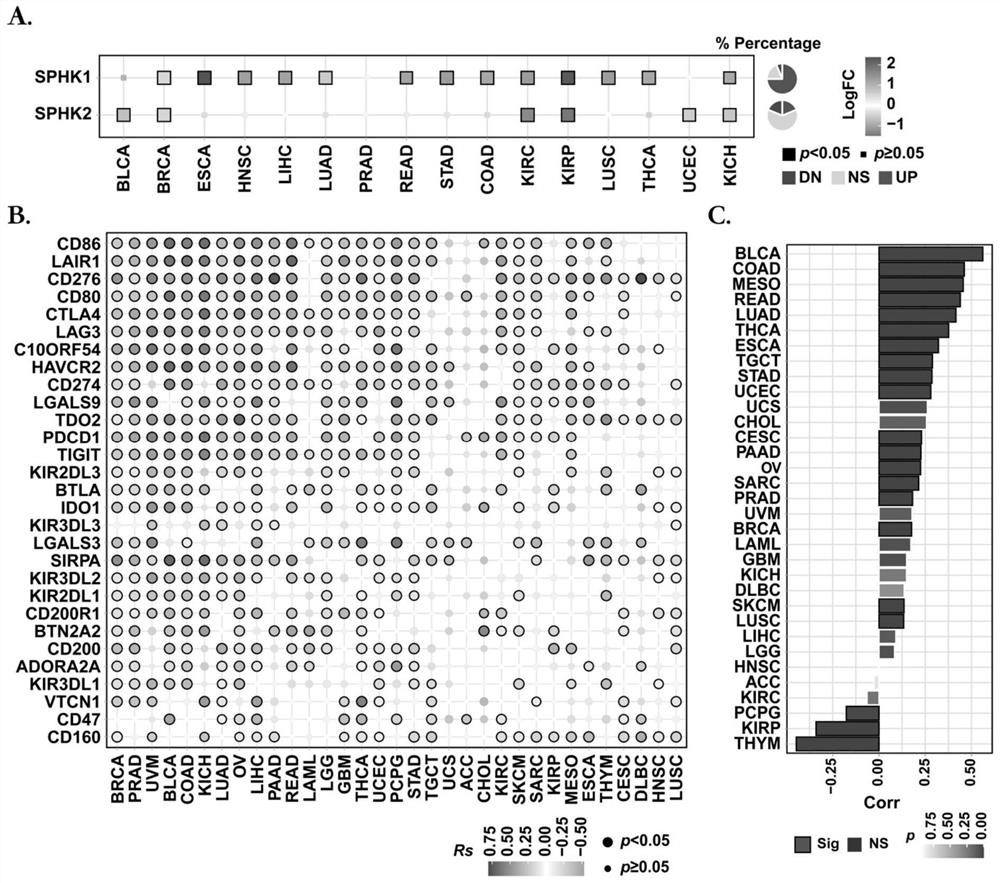
[0037] 1. Expression and function analysis of sphingosine kinase in tumor tissue.
[0038] 1.1 Experimental method: The gene transcriptome of 33 types of cancer (including 10205 tumor samples) was obtained from the Cancer Genome Atlas (TCGA) database (http: / / gdac.broadinstitute.org / ) using the FPKM (Fragments Per Kilobase per Million) algorithm data. Use limma1.4.5 to analyze the fold change (Fold change, FC) of genes in tumors and corresponding normal tissues, and the screening threshold is |Log 2 (FC)|>0.58, using the Benjamini-Hochberg (BH) method to correct the P value of the hypothesis test probability. The Spearman correlation coefficient and its significance test were used to analyze the correlation between genes in tumor tissues, and the test level was α=0.05.
[0039] 1.2 Experimental results:
[0040] Gene set variation analysis was performed by comparing tumor and normal tissues of different cancer types in The Cancer Genome Atlas (TCGA) public database, and the ...
Embodiment 2
[0066] 1. In vitro study of SPHK1-MTA3 axis regulating the expression level of tumor PD-L1.
[0067] 1.1 Experimental methods: transcriptome sequencing, bioinformatics analysis of TCGA database, gene editing, cell gene intervention, PF543 drug intervention, Western blot, ChIP detection
[0068] The target gene siRNA Oligo sequence is shown in Table 2:
[0069]
[0070]
[0071] The specificity of the primers was judged according to the amplification curve, and the gene primers with poor specificity needed to be redesigned and synthesized. The optimized gene primer sequences are shown in Table 3 below:
[0072]
[0073] 1.2 Experimental results:
[0074] See Figure 4 A, Compared with the control group, among the 4182 significantly down-regulated genes detected in the treatment group, nine candidate transcription factors were identified according to the gene differential expression fold;
[0075] See Figure 4 B, In order to evaluate the reliability of the sequenc...
Embodiment 3
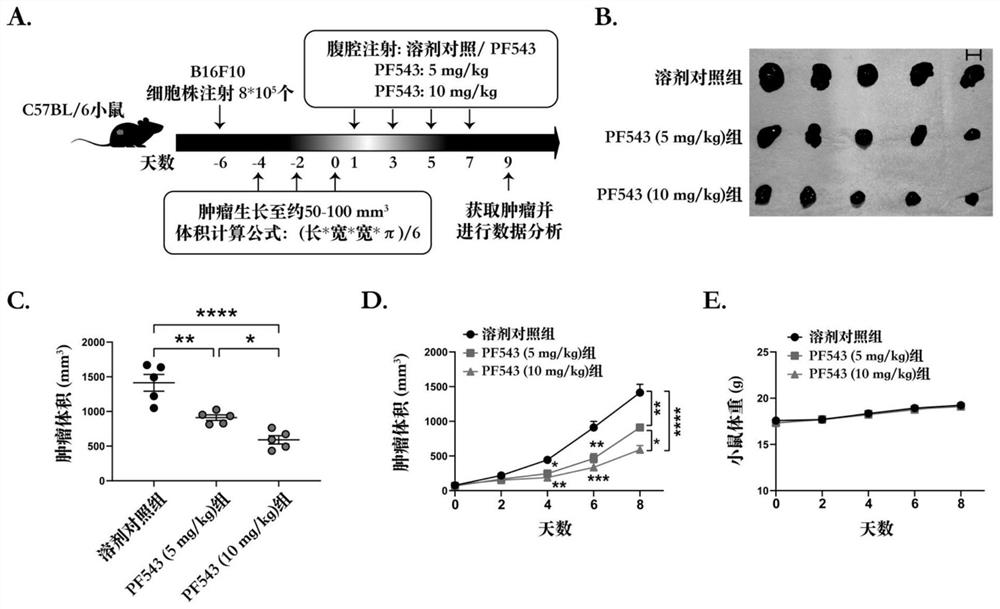
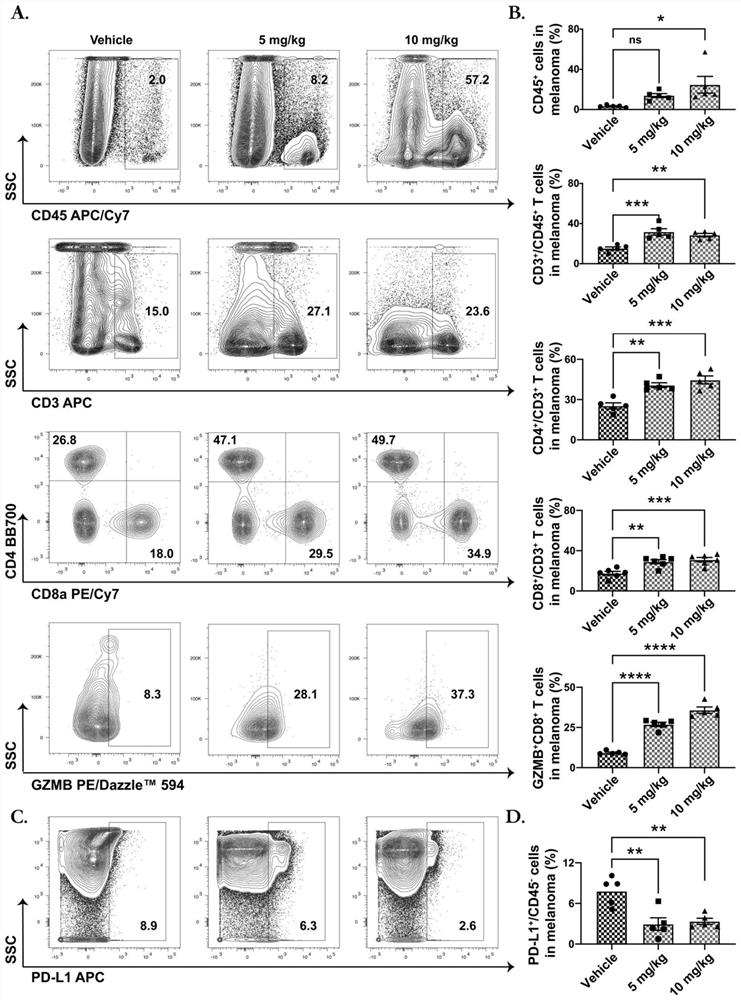
[0093] 1. In vivo study of tumor PD-L1 expression induced by SPHK1 and MTA3.
[0094] 1.1 Group settings:
[0095] CTL+IgG2α isotype control group (control group)
[0096] CTL+PD-1 monoclonal antibody group (PD-1 monoclonal antibody treatment group)
[0097] SPHK1-OE+IgG2α isotype control group (SPHK1 overexpression group)
[0098] SPHK1-OE+PD-1 monoclonal antibody group (SPHK1 overexpression PD-1 monoclonal antibody group)
[0099] MTA3-OE+IgG2α isotype control group (MTA3 overexpression group)
[0100] MTA3-OE+PD-1 monoclonal antibody group (MTA3 overexpression PD-1 monoclonal antibody group)
[0101] 1.2 Experimental process, see Image 6 A:
[0102] About 6 days before the experiment: 6-8 weeks C57BL / 6 mice were subcutaneously injected with B16F10 melanoma SPHK1 overexpression cell line and MTA3 overexpression cell line 8*10 in the right dorsal wing respectively. 5 20 mice each and the same number of CTL cell lines (empty control group).
[0103] The SPHK1 gene ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com