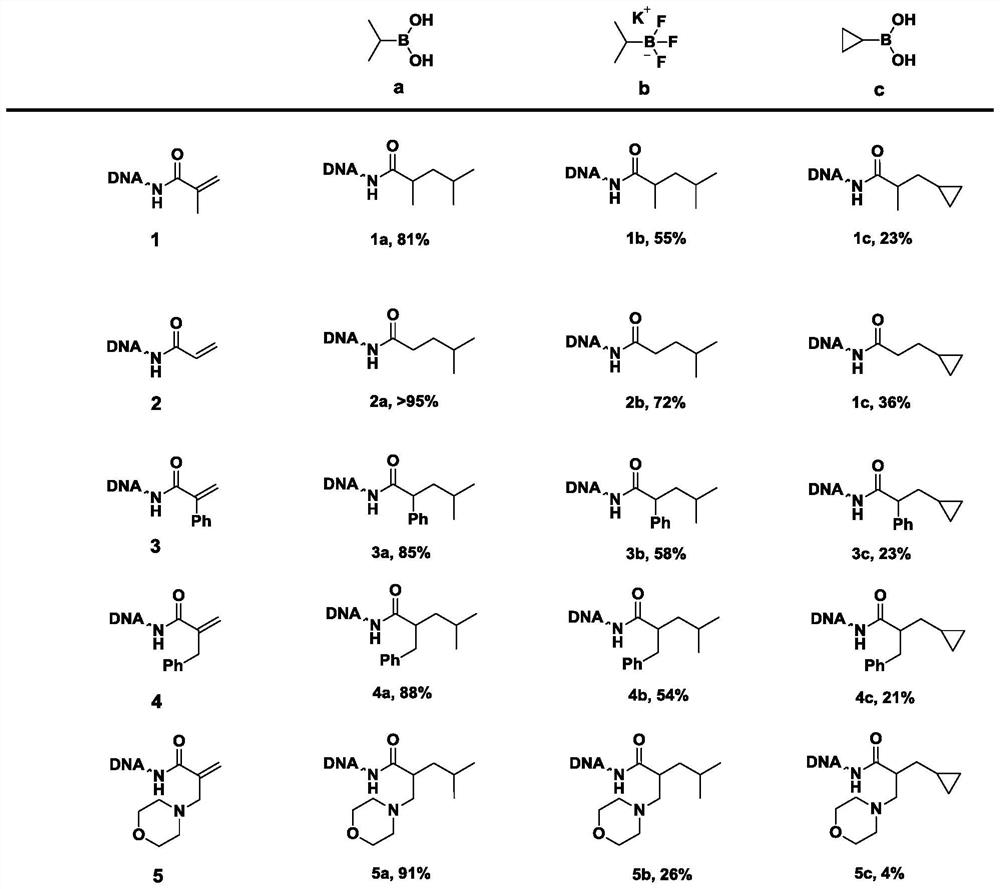
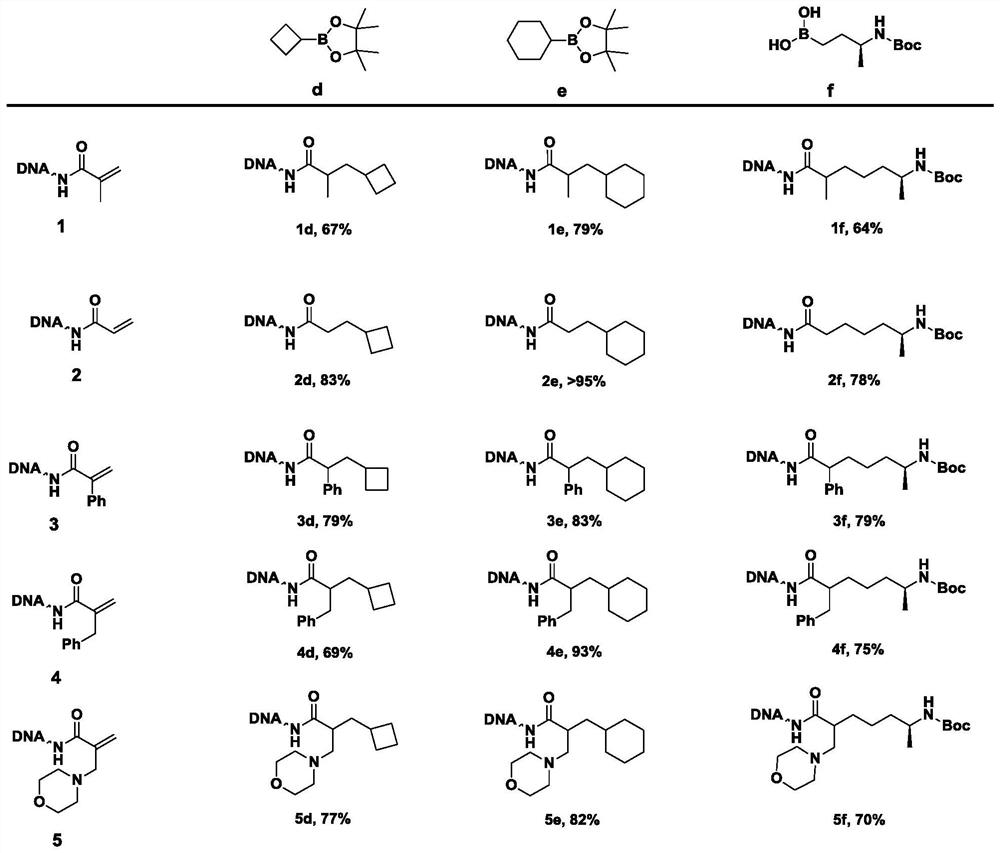
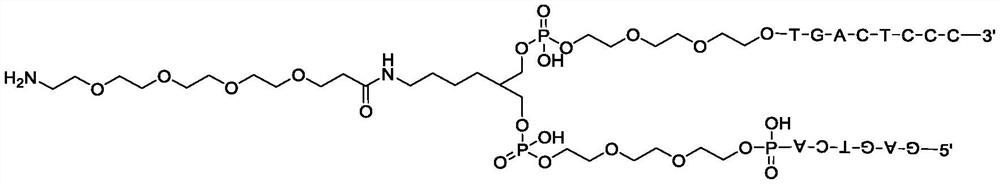
Method for constructing beta-fat substituted ketone compound through On-DNA reaction
A technology for substituted ketones and compounds, applied in the field of constructing On-DNAβ-fatty substituted ketone compounds, can solve problems such as no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the synthesis of On-DNA β-fat substituted ketones
[0057] Step 1. Synthesis of On-DNA α, β-unsaturated carbonyl compounds
[0058]
[0059] DNA-NH 2 (A) Dissolved in 250mM boric acid buffer solution with pH=9.4, and prepared as 1mM concentration solution (20μL, 20nmol); 2-methacrylic acid (25 equivalents, 500nmol, 0.2M DMA solution) and DIPEA (25 equivalents , 500nmol, 0.2M DMA solution) and mix well, then add HATU (25 equivalents, 500nmol, 0.2M DMA solution), mix well, activate at 0°C for 5 minutes; add the activation solution to the DNA solution twice, First react at 0°C for 5 minutes, then place at 25°C for 30 minutes.
[0060] After the reaction, carry out ethanol precipitation: add a total volume of 10% 5M sodium chloride solution to the reacted solution, then continue to add 3 times the total volume of absolute ethanol, shake evenly, and place the reaction in dry ice to freeze 0.5 hours, then centrifuged at 12000rpm at low temperature (4°C) fo...
Embodiment 2
[0065] Embodiment 2, the synthesis of On-DNA β-fat substituted ketones
[0066] According to the preparation method of Example 1, keeping other conditions unchanged, 5 kinds of α, β-unsaturated carbonyl compounds (1-5) were mixed with 6 kinds of fatty boric acids / boric acid esters or trifluoroborates (a-f) respectively Reaction, concrete reaction product productive rate sees accompanying drawing.
[0067]In summary, the present invention controls the solvent, temperature, pH and other conditions during the reaction. In the presence of alkali, On-DNA α, β-unsaturated carbonyl compound and fatty boric acid / boric acid ester or trifluoroboric acid Salt reaction can get On-DNA β-fat substituted ketones. The method has a wide range of substrates, can be carried out in the mixed water phase of organic solvent / water phase, is simple to operate, and is environmentally friendly, and is suitable for the synthesis of a DNA-encoded compound library using a multi-well plate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com