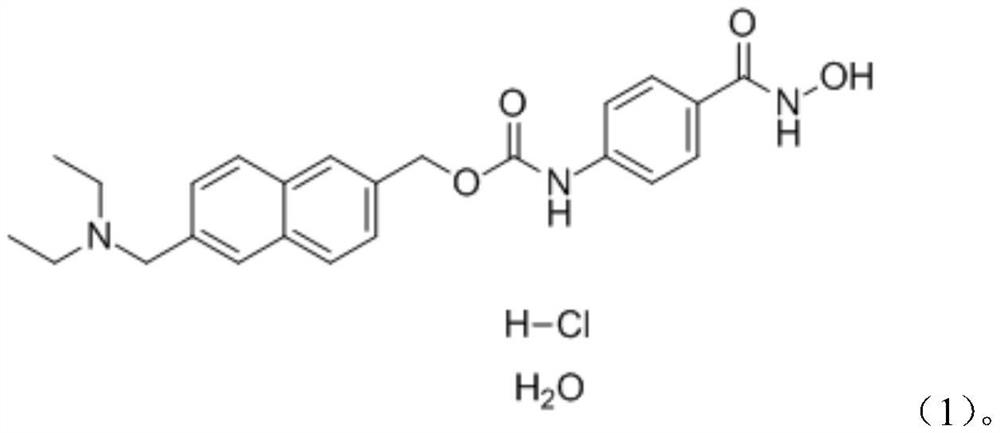
Application of ITF2357 in preparation of medicine for preventing and treating coronavirus
A technology of ITF2357, 1.ITF2357, used in antiviral agents, drug combinations, pharmaceutical formulations, etc., can solve problems such as poor efficacy, and achieve the effects of low effective concentration, increased effect, and high therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
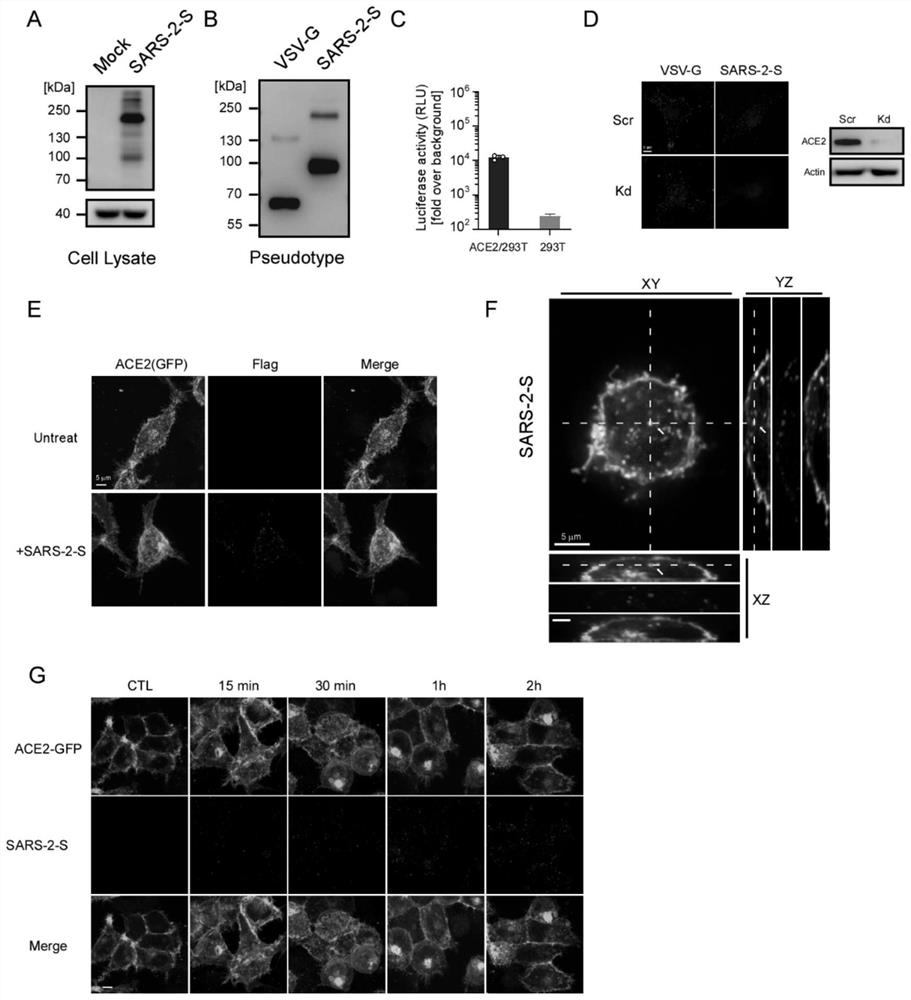
[0035] Example 1 Construction, Production and Verification of Pseudovirus Particles Modified by New Coronavirus S Protein
[0036] In order to simulate the natural process of SARS-CoV-2 using the S protein to recognize the ACE2 receptor to infect host cells, a pseudovirus with a replication-deficient lentivirus as the core and modified with the SARS-2-S protein was constructed particles.
[0037] The construction method adopts the article Xiuyuan Ou et al. (published on March 27, 2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature communications, 11(1), 1 -12. Published method.
[0038] First, the DNA sequence of the SARS-2-S protein was modified without changing its amino acid sequence by means of codon optimization, which facilitated the massive expression of SARS-2-S in 293T cells ( figure 1 A). Subsequently, using the replication-deficient HIV lentivirus commonly used in the laboratory as the c...
Embodiment 2
[0040] Example 2 Cell Biology Platform Screening Clinical Drugs to Inhibit SARS-2-S Pseudovirus Particle Infection
[0041] Utilize the SARS-2-S pseudovirion in vitro infection model (Luciferase reporter system and immunofluorescence staining localization system) constructed in Example 1 to carry out ITF2357 in vitro cell biology verification. ACE2-GFP stably transfected 293T cells were inoculated in 96-well plates, and the cells were pretreated with different concentrations of ITF2357 drugs for 2 hours, and then added with SARS-2-S pseudovirion particles to infect for 3 hours, and then the supernatant was removed and replaced with fresh complete medium. After 40 hours of infection, the cells were lysed using the Luciferase Assay System (Promega) and the reaction substrate was added. Glomax 96 was used to measure the luciferase luminescence intensity, which is proportional to the infection efficiency of virus particles.
[0042] The results of Luciferase activity detection sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com