Racemic 6-methyl nicotine as well as preparation method and application thereof
A technology of methyl nicotine and methyl nicotinic acid, applied in the application, tobacco, tobacco processing and other directions, can solve the problems of unstable production and supply, influence, poor sensory experience, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
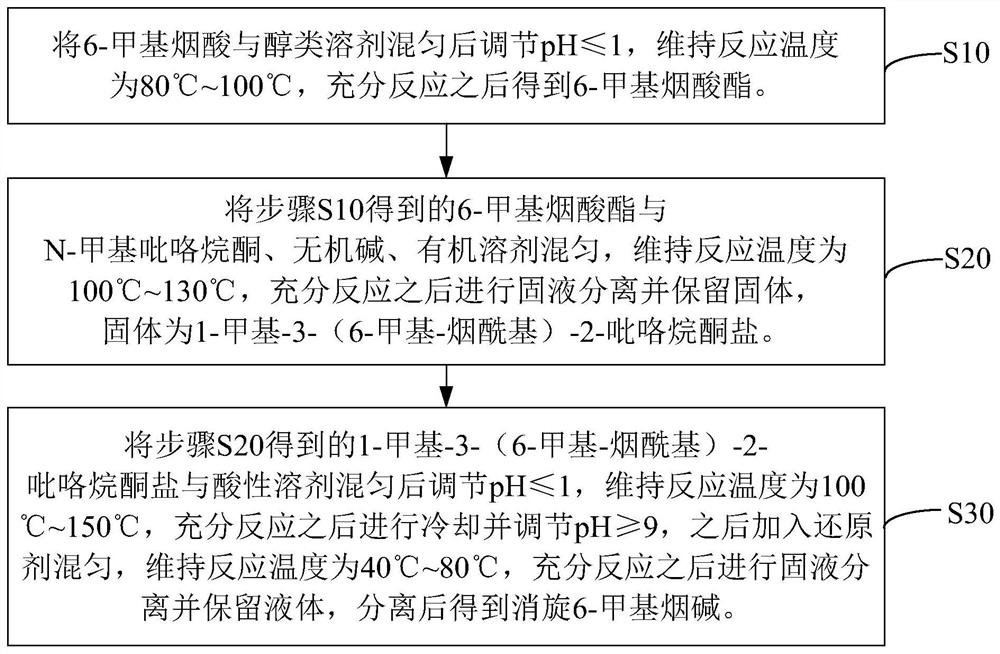
[0037] See figure 1 , the preparation method of the racemic 6-methylnicotine of one embodiment of the present invention, comprises the following steps:
[0038] S10. Mix 6-methylnicotinic acid and an alcoholic solvent, adjust the pH to ≤1, maintain the reaction temperature at 80° C. to 100° C., and obtain 6-methylnicotinic acid ester after sufficient reaction.
[0039] The preparation process of 6-methylnicotinate in step S10 is as follows:
[0040]
[0041] As shown in the above reaction equation, in step S10, 6-methylnicotinic acid and an alcoholic solvent undergo an esterification reaction under acidic conditions to generate 6-methylnicotinic acid ester.
[0042] Wherein, in the operation of maintaining the reaction temperature at 80° C. to 100° C. for sufficient reaction, 6-methylnicotinic acid and alcohol solvents can be heated to reflux under acidic conditions, and the heating and reflux time is determined according to the actual situation.
[0043] In one embodimen...
Embodiment 1
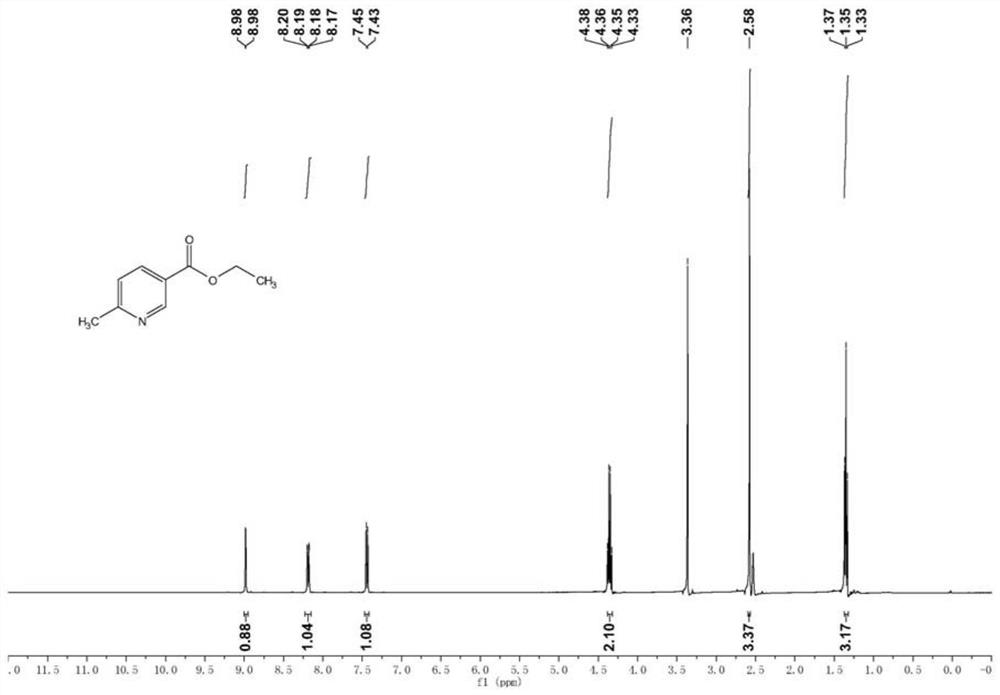
[0096] (1) The preparation process of 6-methylnicotinate is as follows:
[0097]
[0098] Add 400g of 6-methylnicotinic acid into a 5L reaction flask, then add 2L of absolute ethanol, then add 400g of thionyl chloride, and then add 1ml of DMF (dimethylformamide), reflux at 95°C for 4h, pass LC-MS monitors the completion of the reaction. After the reaction is completed, stop heating, cool down, recover the excess solvent and thionyl chloride under reduced pressure, then add sodium bicarbonate solution to adjust the pH to 8, add twice the volume of dichloromethane to extract twice , and then recover the solvent under reduced pressure to obtain ethyl 6-methylnicotinate with a yield of 90%.
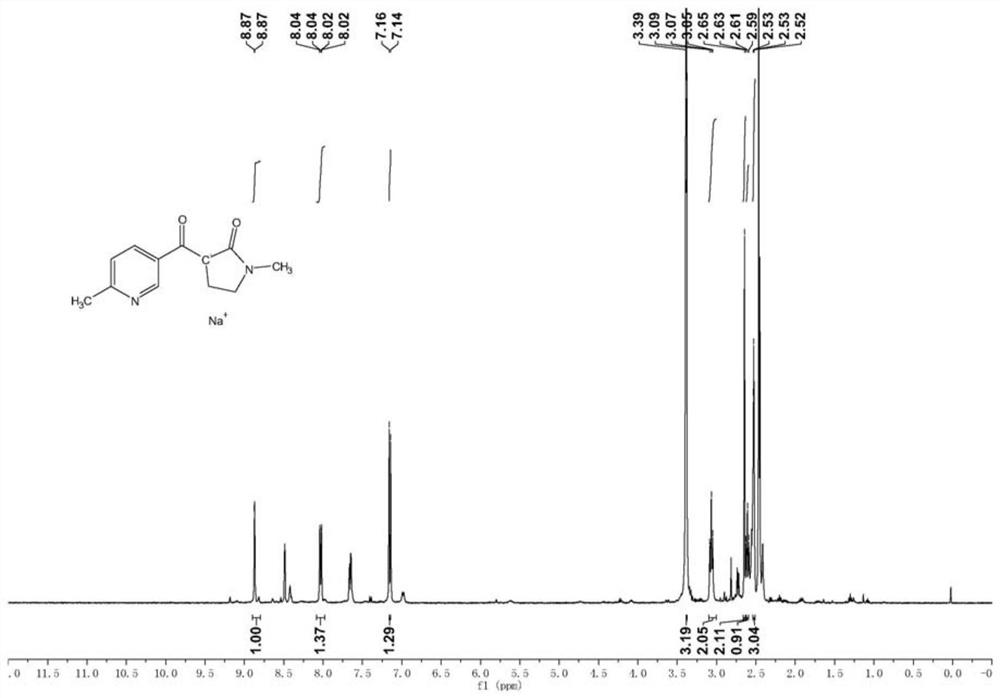
[0099] (2) The preparation process of 1-methyl-3-(6-methyl-nicotinoyl)-2-pyrrolidone salt is as follows:
[0100]
[0101] Take 360g of ethyl 6-methylnicotinate obtained from the above reaction, 300g of sodium tert-butoxide and 300g of N-methylpyrrolidone, dissolve them in 2.5L of tolu...
Embodiment 2~ Embodiment 7
[0110] The preparation method of the electronic smog liquid in Examples 2 to 7 is as follows:
[0111] Take 2ml of propylene glycol and 2ml of glycerol (volume ratio: 5:5) and mix them evenly as a solvent, add 10mg, 20mg, 30mg, 50mg, 70mg, 90mg of the racemic 6-methylnicotine prepared in Example 1, Get different concentrations of racemic 6-methylnicotine solutions; then continue to take a mixed solvent of propylene glycol and glycerol (volume ratio of 5:5) to replenish the volume of different concentrations of racemic 6-methylnicotine solutions Make up to 10ml, and mix evenly on the shaker to obtain electronic cigarettes with racemic 6-methylnicotine concentrations of 1mg / ml, 2mg / ml, 3mg / ml, 5mg / ml, 7mg / ml, and 9mg / ml respectively. Liquids are the electronic cigarette liquids of Embodiment 2 to Embodiment 7 in sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com