Synthesis method of thioketone
A synthesis method and thione technology, applied in organic chemistry and other directions, can solve the problems of low equipment utilization rate, low yield, poor product stability, etc., and achieve the effects of high equipment utilization rate, simple operation steps and high stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
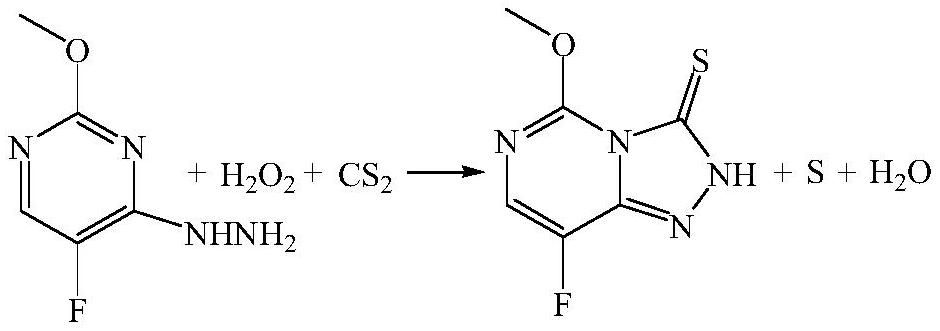
[0028] The synthesis method of 8-fluoro-5-methoxy-2H-[1,2,4]triazolo[4,3-c]pyrimidine-3-thione in this example has the following steps:
[0029] (1) Transfer 1400Kg of dry methanol into the 3000L reactor through a flow meter, open the manhole and put in 600Kg of 5-fluoro-4-hydrazino-2-methoxypyrimidine, heat up to 30°C, stir for 1 hour to completely dissolve and set aside , to detect the content of 5-fluoro-4-hydrazino-2-methoxypyrimidine;
[0030] (2) The reaction temperature of the microchannel reactor I is set to 50 DEG C, and the two channels of the microchannel reactor I are transported with 5-fluoro-4-hydrazino-2-methoxypyrimidine and Carbon disulfide, adjust the flow rate of the diaphragm metering pump to ensure that the mol ratio of 5-fluoro-4-hydrazino-2-methoxypyrimidine to carbon disulfide is 1:1.1, and the residence time of the material in the microchannel reactor I is 5min. After completion, the feed liquid enters the intermediate storage tank for use;
[0031] ...
Embodiment 2
[0034] The synthesis method of 8-fluoro-5-methoxy-2H-[1,2,4]triazolo[4,3-c]pyrimidine-3-thione in this example has the following steps:
[0035] (1) Transfer 1400Kg of dry chloroform into a 3000L reactor through a flow meter, open the manhole and put in 600Kg of 5-fluoro-4-hydrazino-2-methoxypyrimidine, heat up to 30°C, stir for 1 hour to completely dissolve and set aside , to detect the content of 5-fluoro-4-hydrazino-2-methoxypyrimidine;
[0036] (2) The reaction temperature of the microchannel reactor I is set to 60 DEG C, and the two channels of the microchannel reactor I are transported with 5-fluoro-4-hydrazino-2-methoxypyrimidine and Carbon disulfide, adjust the flow rate of the diaphragm metering pump to ensure that the mol ratio of 5-fluoro-4-hydrazino-2-methoxypyrimidine to carbon disulfide is 1:1.2, and the residence time of the material in the microchannel reactor Ⅰ is 6min. After completion, the feed liquid enters the intermediate storage tank for use;
[0037] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com