HPV (human papillomavirus) antigen epitope as well as identification method and application thereof
A technology of HPV16E7 and antigen, which is applied in the fields of cancer antigen components, chemical instruments and methods, and vertebrate antigen components, etc. It can solve the problems that HPV patients cannot be cured, the development and clinical application of HPV therapeutic vaccines are urgent, and the infection cannot be cleared.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0073] According to some specific embodiments of the present invention, the HPV antigen epitope includes at least one of the amino acid sequences shown in SEQ ID NO: 1-7.
[0074] In yet another aspect, the present invention provides a mutant, which has at least one mutation site in addition to the anchor site, compared to the aforementioned isolated peptide (HPV E6, SEQ ID NO: 1). According to a specific embodiment of the present invention, when the obtained HPV antigenic epitope is an octapeptide, the 2nd and 8th amino acids of the obtained HPV antigenic epitope are the anchor sites of HLA, and the amino acids at other sites in the epitope are After substitution, for example, the E6 HPV epitope in this application is mutated at the first, third, fifth, sixth, and seventh positions, and it can still have the same or related immunogenicity and Its potential therapeutic effect is that it can be presented by HLA-I molecules, recognized by CTL cells or TIL cells, and then present...
Embodiment 1
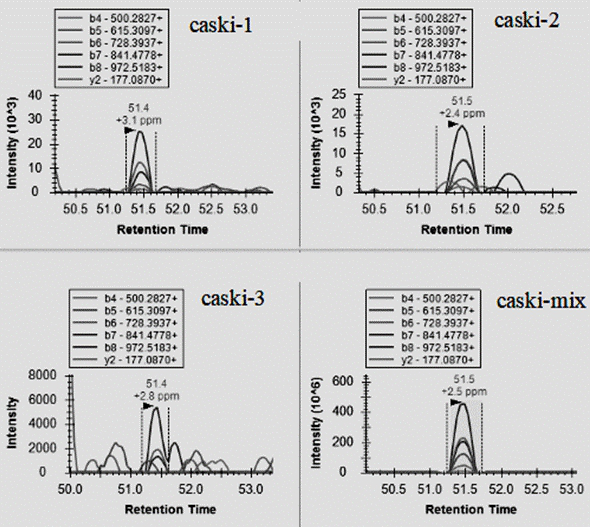
[0159] Example 1 Identification of HPV antigenic epitopes
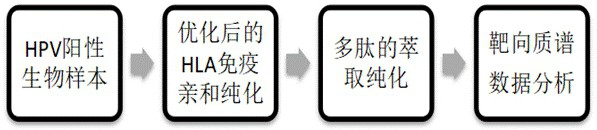
[0160] In this example, the immunoaffinity method is used to obtain the immune polypeptide, and the specific operation process is as follows: figure 2 As shown, the specific operation steps are as follows:
[0161] 1.1 Acquisition of HLA protein complexes (major histocompatibility complex (MHC) class I complex molecules)
[0162] The specific experimental operation of this experiment is as follows:
[0163] 1) Take 1mL protein A Sepharose CL-4B (GE healthcare) 50% resin into Poly-PrepChromatography Columns, flow through ultrapure water and PBS to wash, and flow through 4mg anti-HLA-class A, B, C antibodies ( W6 / 32, ATCC HB-95);
[0164] 2) After flowing through PBS, flow through cross-linking equilibrium solution (200mM triethanolamine);
[0165] 3) Flow through the cross-linking reaction solution, retain 1mL of the cross-linking reaction solution (50mM DMP), close the lower channel, and let it stand at room temp...
Embodiment 2
[0194] Example 2 Evaluation of HPV-specific tumor antigen peptide immunogenicity by enzyme-linked immunospot assay (ELISPOT)
[0195] In the experiment, T2 cells were loaded with the newly identified 7 kinds of HPV16 type-specific mixed antigen polypeptides described in Example 1, which were presented to cytotoxic T cell CTL through the antigen presentation function of T2 cells, and TAP (antigen presentation and transport) of T2 cells Molecular defects activate immune responses upon presentation of experimental antigens by payloads of exogenous polypeptides. The experiment set up positive control group, negative control group and test product group. After the living cells of T2 (ATCC: CRL-1992) in the logarithmic growth phase were collected after cell culture treatment, they were diluted with medium and resuspended to 5e 5 cells / mL for later use. Take 1mL T2 cell group from each group, add phytohemagglutinin PHA (Sigma, product number L8902-25MG) to the positive control grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com