A method for preparing HPV antigen-specific cytotoxic T lymphocytes
A cytotoxic, lymphocyte technology, applied in the field of biotechnology development and application research, can solve the problems of limited materials, low cell expansion efficiency, long preparation cycle, etc., to enhance antigen presentation, prolong survival time, stimulate T cells effect of proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
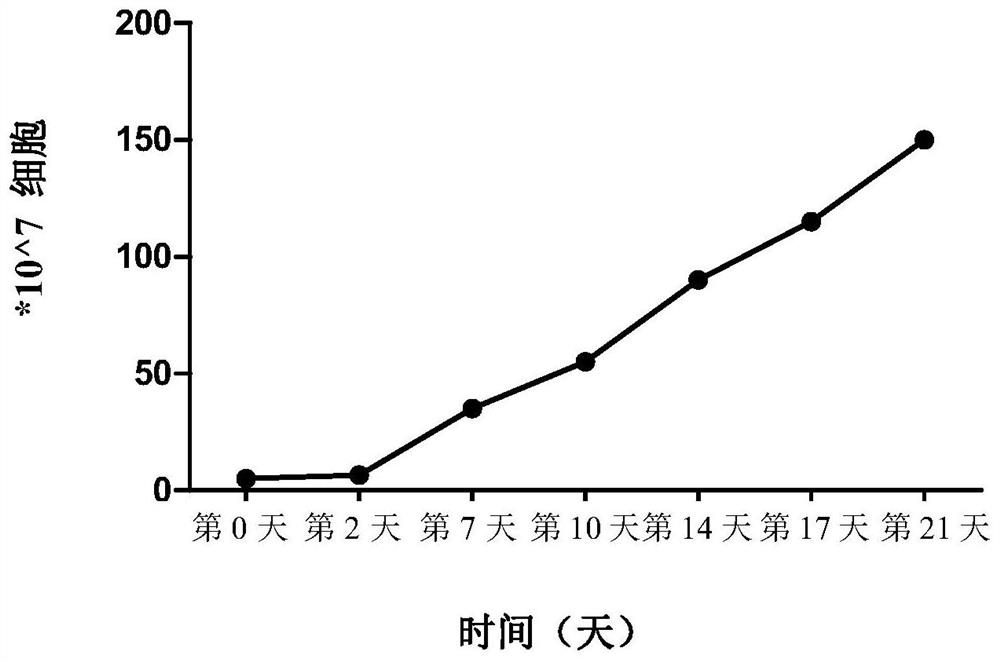
[0043] Example 1: Preparation of HLA-A2402-restricted anti-HPV antigen-specific CTL
[0044] (1) HLA-A2402 + Typing test: collect 2mL of blood from the subject (EDTA anticoagulant), and send it out for testing of HLA typing (Beijing Boao Jingdian Biotechnology Co., Ltd.).
[0045] (2) Synthesis of antigenic peptide: HPV16E6 antigenic peptide, the site is 49-57, and the sequence is 9 peptides of VYDFAFRDL (SEQ IDNO: 1, hereinafter referred to as HPV16E6 49-57 Peptide), chemically synthesized (Shanghai Gill Biochemical Co., Ltd.), fully dissolved in sterile double distilled water, the peptide concentration was 2mg / ml, and stored in -80°C in aliquots.
[0046] (3) Peripheral blood collection and peripheral blood mononuclear cell (PBMC) isolation: 50 mL of peripheral venous blood was collected in a vacuum blood collection tube anticoagulated with heparin, and PBMC were obtained after Ficoll density gradient centrifugation:
[0047] a. Transfer the collected blood sample to a 50m...
Embodiment 2
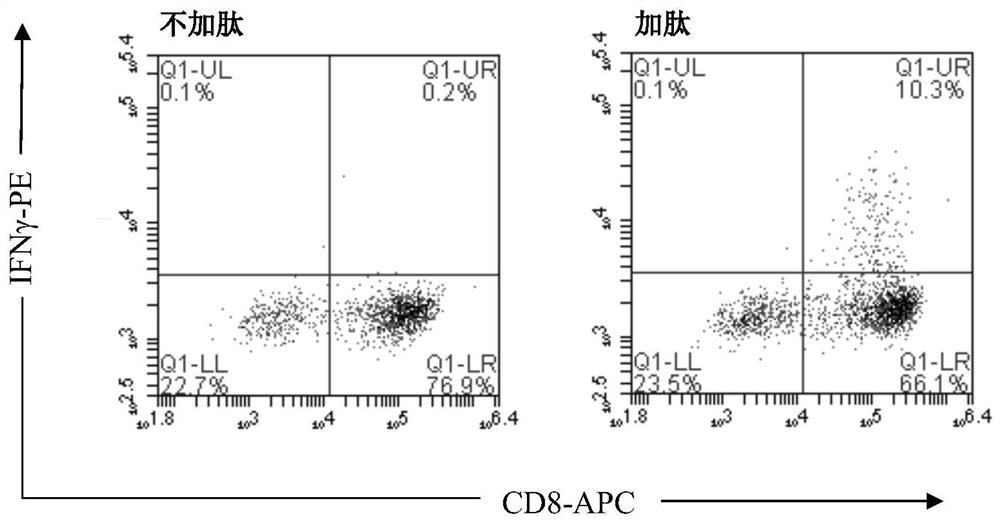
[0056] Example 2: Detection of CD8 after cell expansion by flow cytometry + / IFNγ + cell ratio
[0057] (1) Get the cell sample obtained on the 14th day of cultivation in Example 1, 1×10 6 Cells / tube, wash 2 times with PBS.
[0058] (2) Add antigenic peptide (HPV16E6 49-57 Peptide, Shanghai Golgi Biochemical Co., Ltd.) and protein transport inhibitor Golgi-stop (BD, product number 554715) stimulated and activated cells, and incubated in a cell culture incubator for 3h.
[0059] (3) Flow detection antibody was added for flow detection: human CD8-APC antibody (BD, Cat. No. 555369), incubated at room temperature in the dark for 30 min, washed twice with PBS.
[0060] (4) Add fixed permeabilization solution (BD, Cat. No. 554715), incubate at room temperature in the dark for 30 min, and wash twice with PBS.
[0061] (5) Add flow detection antibody for flow detection of intracellular factors: human IFNγ-PE antibody (BD, Cat. No. 559327), incubate at room temperature in the dark...
Embodiment 3
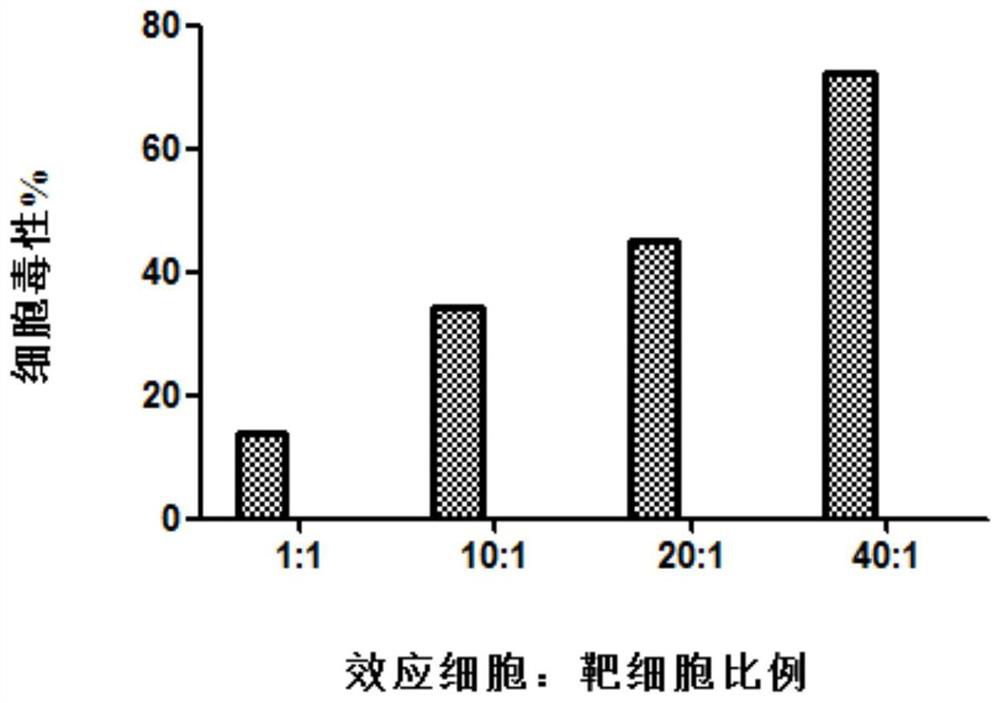
[0064] Embodiment 3: Detection of cell killing activity by lactate dehydrogenase (LDH) method
[0065] Take the Caski cell line in the logarithmic growth phase (purchased from the China Institute for Food and Drug Control) as the target cells, and adjust the cell density to 3 × 10 4 cells / mL, 100 μL per well was spread in a 96-well culture plate. Take the CTL cells obtained in Example 1 of the present invention on the 14th day and adjust the density to 3×10 4 pcs / mL, 3×10 5 pcs / mL, 6×10 5 pcs / mL, 1.2×10 6 1 / mL, added to a 96-well culture plate, 100 μL per well, so that the effect-to-target ratios were 1:1, 10:1, 20:1, and 40:1, respectively, and three replicate wells were set up for each group.
[0066] (1) Analysis board settings
[0067] a. Spontaneous LDH release by effector cells: different effector-target ratios, take the corresponding number of effector cells and add them to the reaction plate;
[0068] b. Experimental well: cells with different effect target ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com