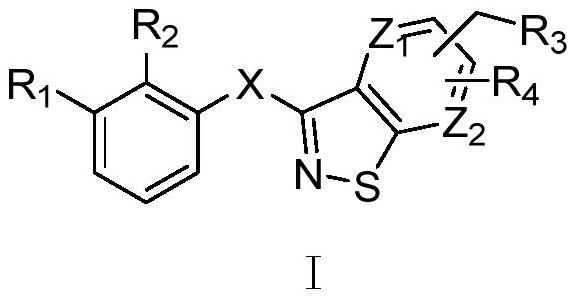
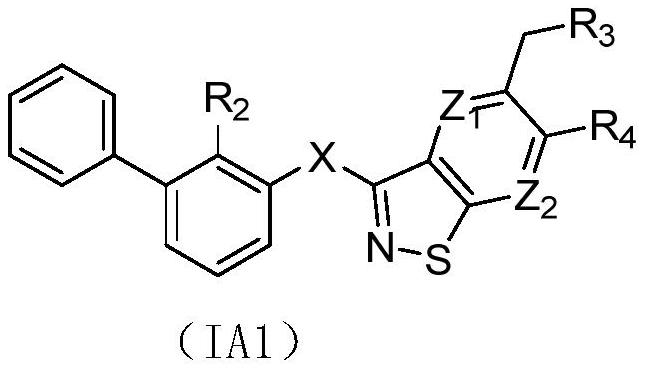
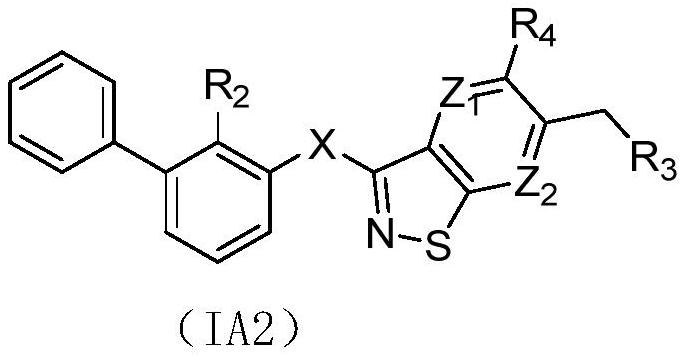
Isothiazole heterocyclic compound, and preparation method, pharmaceutical composition and application thereof
A technology of isothiazoles and compounds, applied in the field of medicinal chemistry, can solve the problems of being unable to take orally, only injecting or dripping, unstable in the body, etc., and achieve the effect of inhibiting growth and high inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0502] Example 1: 1-(3-(2-chloro-3-(1,4-benzodioxan-6-yl)anilino)isothiazolo[4,5-b]pyrazine-6-ylidene Methyl)pyrrolidin-3-ol
[0503]
[0504] 3-Hydroxy-5-methylpyrazine-2-carboxamide:
[0505] In the 100mL reaction flask, add methylglyoxal (40% aqueous solution, 21.6ml, 120mmol) and NaHSO 3 (15.6g, 150mmol), start stirring, and then slowly add dropwise NaOH aqueous solution (500mg, 12.5mmol, 39ml water) to the solution, react at 80°C after the dropwise addition, and slowly add 2-aminopropyl Diamide (11.7g, 100mmol), continue to react at 80°C for 3h, stop heating, add NaA after cooling to room temperature C (20.5 g, 250 mmol) and H 2 o 2 (16.4ml), stirred overnight at room temperature. The reaction solution was suction filtered to obtain 9.7 g of pink solid. Yield: 63.4%. 1 H NMR (400MHz, DMSO-d 6 )δ13.40(s,1H),8.64(s,2H),7.39(s,1H),2.21(s,3H).
[0506] 3-Chloro-5-methylpyrazine-2-carbonitrile:
[0507] Add 3-hydroxyl-5-methylpyrazine-2-carboxamide (9.7 g, 63.4 mm...
Embodiment 2
[0518] Example 2: 1-(3-(2-chloro-3-(1,4-benzodioxan-6-yl)anilino)isothiazolo[4,5-b]pyridine-6-methylene base) pyrrolidin-3-ol
[0519]
[0520] 2-cyano-3-nitro-5-methylpyridine:
[0521] Add 2-bromo-3-nitro-5-methylpyridine (1g, 4.61mmol) and CuCN (454mg, 5.07mmol) into a three-necked flask, replace with argon, add 5ml of DMF into the syringe, and react at 70°C for 4h. Cool down to room temperature, add EA / H 2 O (40ml / 20ml), filter with diatomaceous earth, separate liquid, wash the organic phase once with 20ml water, wash once with 20ml ammonium chloride / ammonia water, extract the aqueous phase twice with 20ml EA, combine the organic phases, and wash once with 20ml saturated brine twice, and dried over anhydrous sodium sulfate. It was evaporated to dryness under reduced pressure, and prepared under medium pressure (PE:EA 4:1-2:1) to obtain 347 mg of yellow-white solid. Yield: 46.14%. HRMS(ESI)m / z:164.04453[M+H] + . 1 H NMR (400MHz, DMSO-d 6 )δ8.93(dd, J=1.8,0.7Hz,1H...
Embodiment 3
[0535] Example 3: (S)-1-(3-(2-chloro-3-(1,4-benzodioxan-6-yl)anilino)isothiazolo[4,5-b]pyrazine -6-methylene)pyrrolidin-3-ol
[0536]
[0537] Using (S)-3-hydroxypyrrolidine instead of racemic 3-hydroxypyrrolidine, the operation is the same as in Example 1 to obtain (S)-1-(3-(2-chloro-3-(1,4-benzobis Oxan-6-yl)anilino)isothiazolo[4,5-b]pyrazin-6-methylene)pyrrolidin-3-ol yellow solid. HRMS(ESI)m / z:496.11857[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com