N-methyl-2,3,7,8-tetrahydroxy benzophenanthridine compound, preparation method and application
A hydroxybenzophenanthridine and compound technology, applied in the field of natural medicines, can solve the problems of unsatisfactory DDC inhibitory activity, bond inactivation, etc., and achieve the effect of retaining DDC inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] N-methyl-2,3,7,8-tetrahydroxytriphenylene quaternary ammonium salt (I) preparation (see equation 2):
[0040]
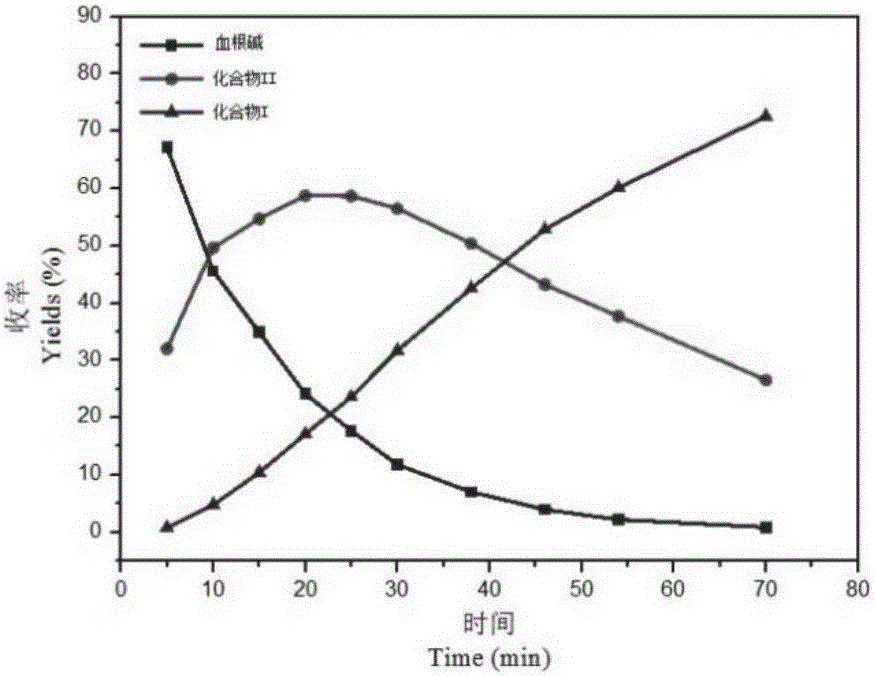
[0041] Accurately weigh 1g of sanguinarine chloride and equivalent phloroglucinol (phloroglucinol), add 30ml of 60% concentrated sulfuric acid, stir at 95°C, and stir at 5, 10, 15, 20, 25, 30, 38, 46, At 54 and 70 minutes, a small amount of reaction solution was dropped into a vial containing a small amount of ultrapure water, and the precipitate and supernatant were collected by centrifugation for HPLC-QTOF detection. Since sanguinarine has two methylenedioxy groups, removing one methylene group will give two phenolic hydroxyl derivatives (II), the reaction time is prolonged, and the conversion rate of the target product I is gradually increased. According to the concentration and time plotting of sanguinarine chloride, the bisulfate of compound I and the bisulfate of compound II in the products of different reaction times, obtain the conversion rate-react...
Embodiment 2
[0043] Separation and purification of N-methyl-2,3,7,8-tetrahydroxytriphenylene phenanthridine quaternary ammonium salt (I)
[0044] The reaction product of Example 1 was added to 200 mL of ultrapure water for centrifugation, the supernatant was discarded, and the precipitate was repeatedly washed with ultrapure water for 3 times to remove sulfuric acid. The precipitate was ultrasonically dissolved with 44% formic acid to prepare a sample solution for later use. Pack the pretreated AB-8 resin into a column with an inner diameter of 4cm, a resin height of 54cm, and a column volume of 1BV=678mL. The top of the resin is protected with absorbent cotton, and the resin column is pre-washed with 1BV44% formic acid at a flow rate of 3BV / h , when the liquid level above the resin column is 5cm away from the absorbent cotton, it is ready to load the sample. The flow rate of the sample is controlled at 2BV / h. Chromatography for detection, liquid chromatography detection conditions are: c...
Embodiment 3
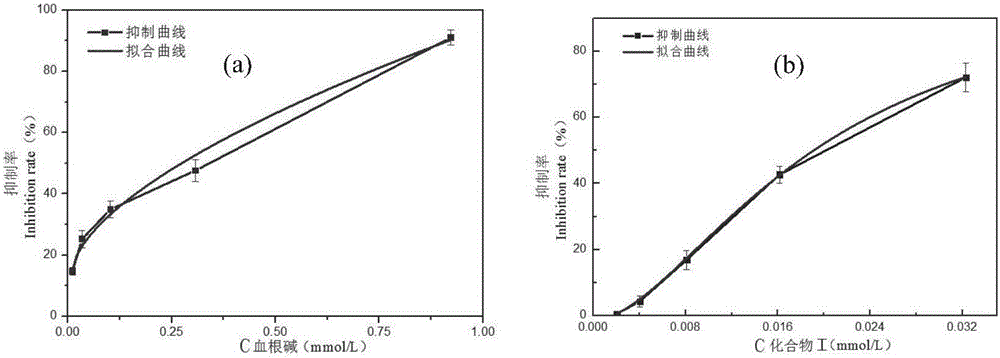
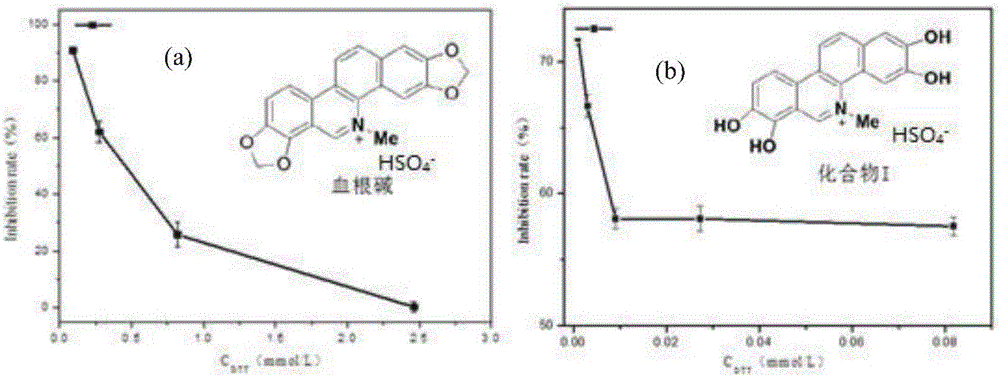
[0051] Research on the inhibitory activity of sanguinarine and compound I (purified according to Example 2) on DDC
[0052] Sanguinarine was formulated as 2.29×10 according to the results of DDC inhibitory activity pre-test -6 mol / ml, the compound I concentration is 3.044×10 -7 mol / ml solution. The remaining components used in the enzymatic reaction system are: phosphate buffer solution is Na 2 HPO 4 -KH 2 PO 4 (0.02M, pH6.8), the concentration of human recombinant dopa decarboxylase (human recombinized DDC) phosphate buffer solution is 10 μg / mL, the concentration of PLP phosphate buffer solution is 5×10 -6 mol / mL, L-Dopa phosphate buffer solution is 2.5×10 -6 mol / ml. Under the premise of ensuring that the total volume of the reaction system is 2 mL and the DDC concentration is 40 ng / uL, 15 μL of PLP solution, phosphate buffer, different concentration gradients of sanguinarine or the phosphate buffer solution of compound (I) and DDC phosphate buffer solution, sealed at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com