Triazine compound or pharmaceutically acceptable salt, isomer, pharmaceutical composition and application thereof
A compound and triazine technology, applied in the field of preparation of triazine compounds, can solve the problems of not finding hERG inhibition, mutagenicity/cleavage and phototoxicity, etc., and achieve lower single dose and better treatment. effect, the effect of increasing blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
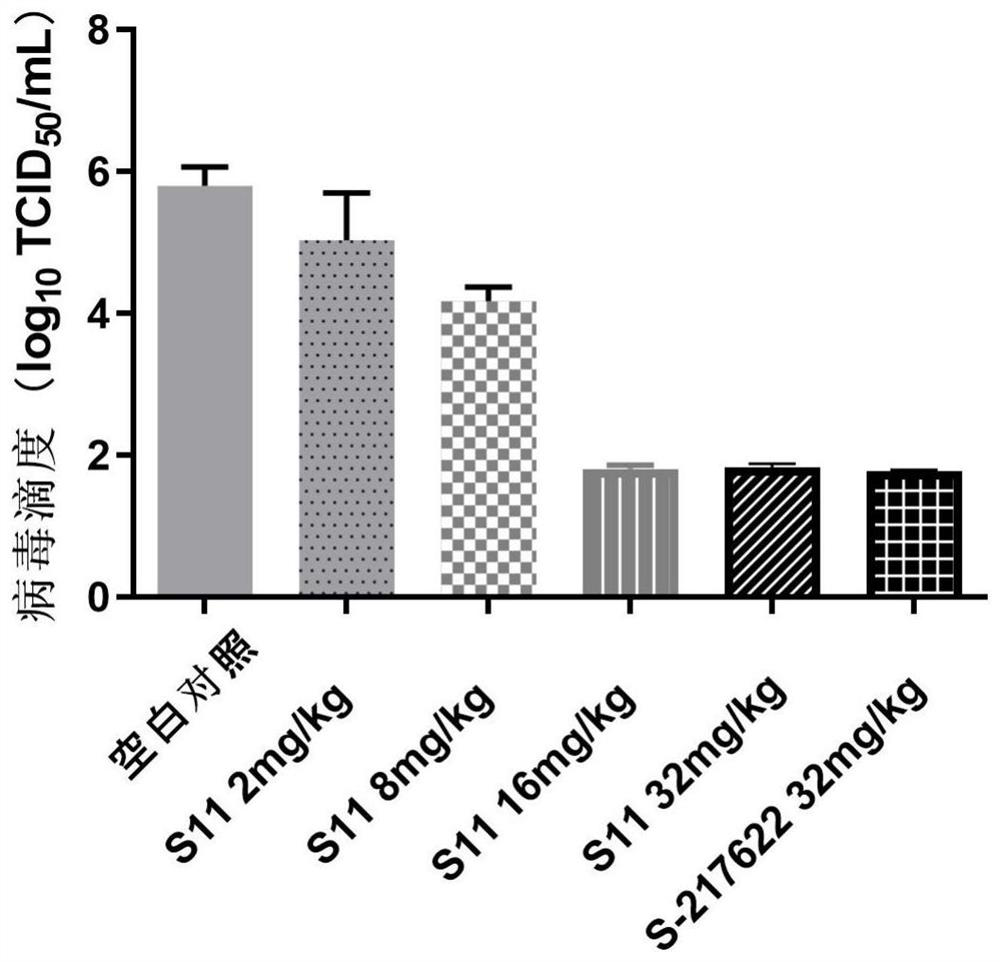
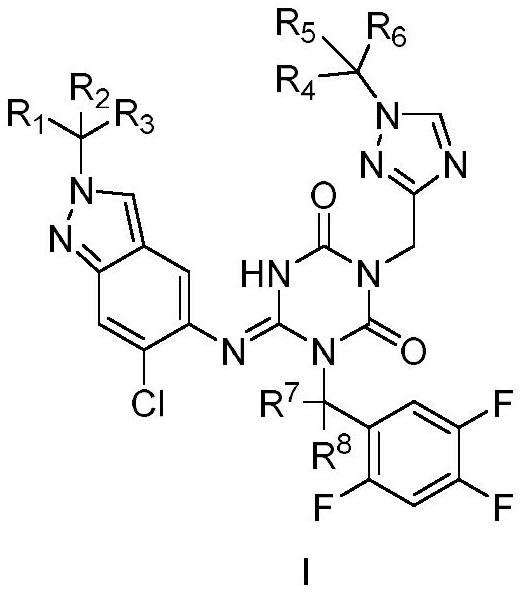
Image
Examples
Embodiment 1
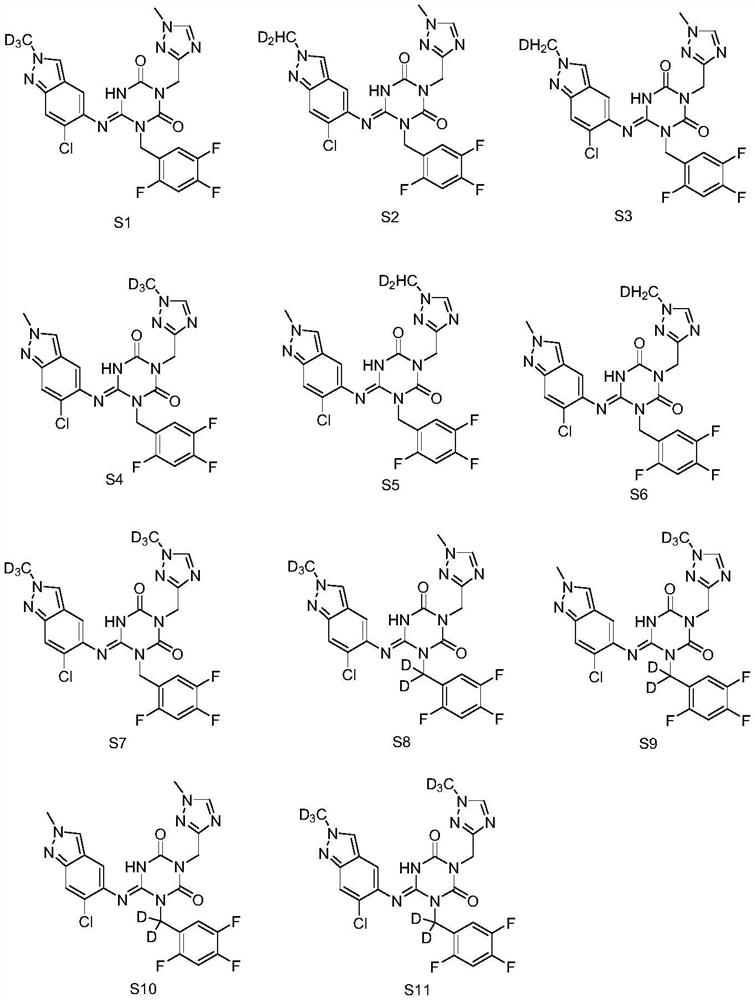
[0046] Example 1: Synthesis of Compound S1
[0047]
[0048] Step 1: Synthesis of deuterated compound 4a
[0049]
[0050] (1) Compound 1 (37.2g, 0.2mol) was suspended in water (190ml) and concentrated hydrochloric acid (50ml, 0.6mol), at 0°C, NaNO 2 Aqueous solution (13.80g, 0.2mol, 32.20mL) was added dropwise to the above solution, then stirred for 30min, filtered, pre-cooled NaBF 4 (24.20 g, 0.22 mol) in an aqueous solution (90 ml) was added to the above filtrate and stirred at 0°C for 40 min. The stirring was stopped, filtered, and the filter cake was washed with cold ethanol and diethyl ether. The filter cake was collected and dried to obtain the diazonium salt (22.5 g, 0.097 mol). Dissolve the diazonium salt in CHCl 3 (231 mL), KOAc (15.15 g, 0.155 mol) was added to the above solution, stirred at room temperature, monitored by TLC, the reaction was complete, and the stirring was stopped. Add water (200ml) to quench the reaction, extract with DCM (100mL × 3), co...
Embodiment 2
[0069] Example 2: Synthesis of Compound S2
[0070]
[0071] The synthesis method is as in Example 1, only need to replace the corresponding raw material. 1 H NMR (300MHz, DMSO-d 6 ,DCl inD 2 O)δ9.32(1H,s),8.42(1H,s),7.77(1H,s),7.46(1H,m),5.36(2H,s),5.08(2H,s),4.18(3H, s),3.94(1H,s).MS(ESI,m / z):534(M + +1).
Embodiment 3
[0072] Example 3: Synthesis of Compound S3
[0073]
[0074] The synthesis method is as in Example 1, only need to replace the corresponding raw material. 1 H NMR (300MHz, DMSO-d 6 ,DCl inD 2 O)δ9.32(1H,s),8.42(1H,s),7.77(1H,s),7.46(1H,m),5.36(2H,s),5.08(2H,s),4.18(3H, s),3.94(2H,s).MS(ESI,m / z):533(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com