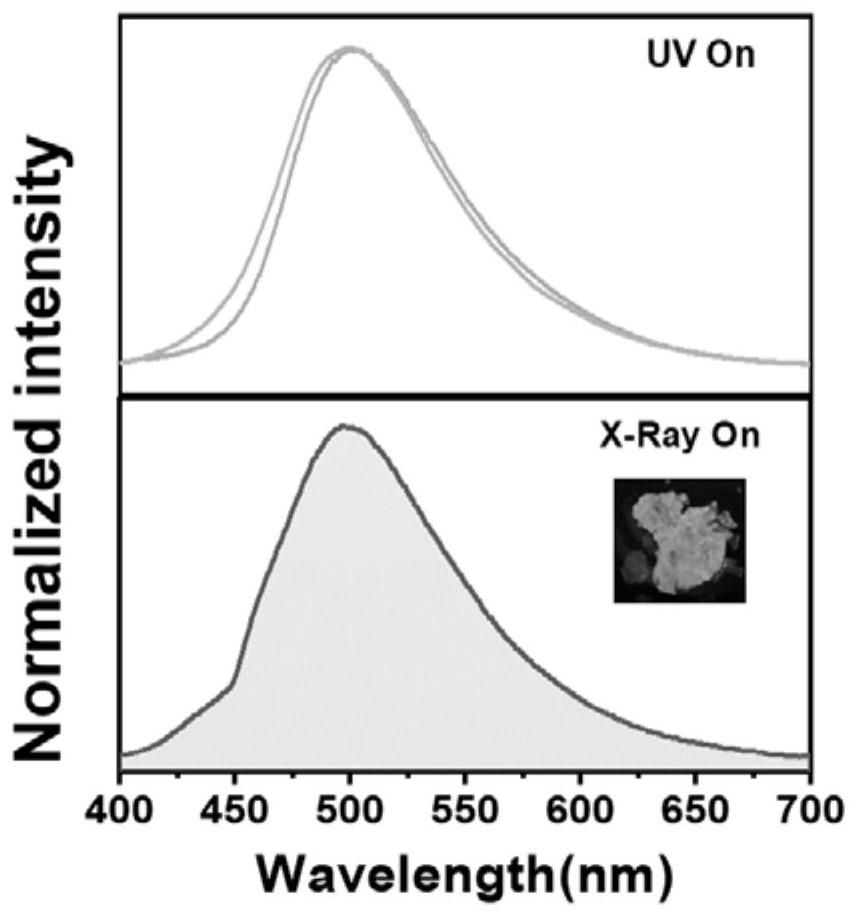
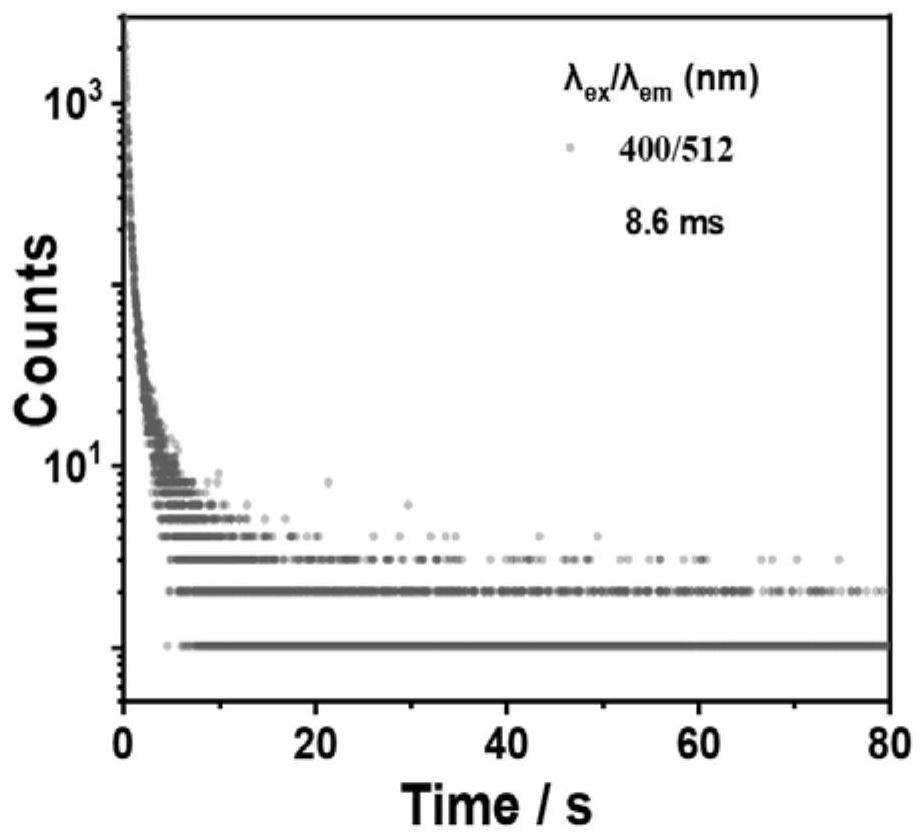
Organic room-temperature phosphorescent polymer, preparation thereof and application of organic room-temperature phosphorescent polymer in X-ray imaging
A room temperature phosphorescence, polymer technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of strict growth conditions, difficult reproducibility, application limitations, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
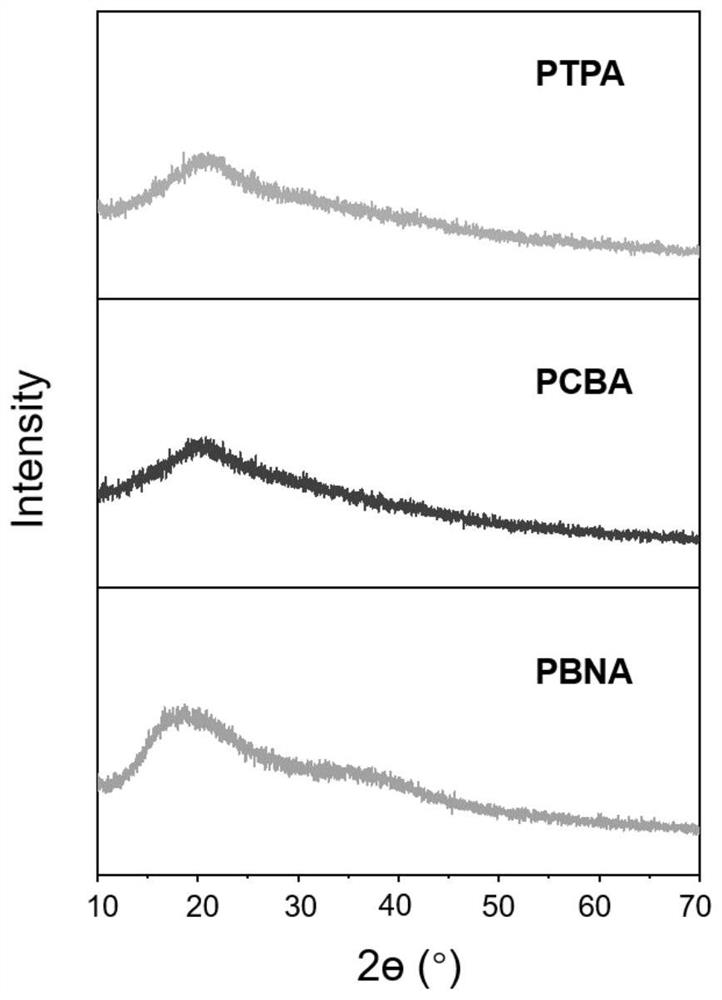
[0035] Example 1: Preparation of PTPA
[0036] The chemical structural formula of PTPA is as follows:
[0037]
[0038] The synthesis of PTPA is divided into three steps:
[0039] 1) Under a nitrogen atmosphere, dissolve 4-bromotriphenylamine (1.0equiv) in an ultra-dry tetrahydrofuran solution, add n-butyllithium (1.2equiv) at -78°C to react for 1 h, and then add diphenylphosphorus chloride ( 1.0equiv) reacted for 12h, spin-dried under reduced pressure, and purified by column chromatography to obtain MT;
[0040] 2) Under a nitrogen atmosphere, dissolve MT (1.0 equiv) in DMF, add 4-bromo-1-butene (2.0 equiv), heat at 120° C. for 18 h, distill under reduced pressure, spin dry, and purify by column chromatography to obtain MTPA;
[0041] Characterization of compound MTPA: 1 H NMR (400MHz, DMSO-d 6 ): δ7.78(s,11H),7.29(s,12H),7.04–6.84(m,2H),5.96–5.74(m,1H),5.39–4.93(m,2H),3.70–3.47(m ,2H),2.38–2.05(m,2H). 13 CNMR (100MHz, DMSO-d 6 ,δ): 162.66, 153.25, 145.09, 135.43, ...
Embodiment 2
[0045] Embodiment 2: the preparation method of PCBA
[0046] The chemical structural formula of PCBA is as follows:
[0047]
[0048] The synthesis of PCBA is divided into three steps:
[0049] 1) Under nitrogen atmosphere, dissolve 3-bromo-9-butanecarbazole (1.0equiv) in ultra-dry tetrahydrofuran solution, add n-butyllithium (1.2equiv) at -78°C to react for 1h, add diphenyl chloride Phosphorus (1.0equiv) was reacted for 12h, spin-dried under reduced pressure, and purified by column chromatography to obtain MC;
[0050] 2) Under nitrogen atmosphere, dissolve MC (1.0 equiv) in DMF, add 4-bromo-1-butene (2.0 equiv), heat at 120° C. for 18 h, spin dry under reduced pressure, and purify by column chromatography to obtain MCBA;
[0051] Characterization of compound MCBA: 1 H NMR (400MHz, DMSO-d6): δ8.89–8.75 (m, 1H), 8.33–8.23 (m, 1H), 7.99–7.68 (m, 14H), 7.38–7.28 (m, 1H), 6.06– 5.80(m,1H), 5.26–5.00(m,2H), 4.51(s,2H), 3.86–3.67(m,2H), 2.41–2.25(m,2H), 1.88–1.68(m,2H), 1....
Embodiment 3
[0055] Example 3: Preparation method of PBNA
[0056] The chemical structural formula of PBNA is as follows:
[0057]
[0058] The synthesis of PCBA is divided into three steps:
[0059] 1) Under a nitrogen atmosphere, dissolve 2,6-dibromonaphthalene (1.0 equiv) in an ultra-dry tetrahydrofuran solution, add n-butyllithium (1.2 equiv) at -78°C to react for 1 h, add diphenylphosphorus chloride ( 1.0equiv) reacted for 12h, spin-dried under reduced pressure, and purified by column chromatography to obtain MB;
[0060] 2) Under nitrogen atmosphere, dissolve MB (1.0 equiv) in DMF, add 4-bromo-1-butene (2.0 equiv), heat at 120° C. for 18 h, spin-dry under reduced pressure, and purify by column chromatography to obtain MBNA ;
[0061] Characterization of compound MBNA: 1 H NMR (400MHz, DMSO-d 6 ): δ8.67–8.54(m,1H),8.53–8.39(m,1H),8.32–8.24(m,1H),8.16–8.11(m,1H),7.94–7.76(m,13H),6.04 –5.80(m,1H),5.27–5.03(m,2H),3.90–3.69(m,2H),2.42–2.22(m,2H). 13 C NMR (100MHz, DMSO-d 6 ,δ):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com