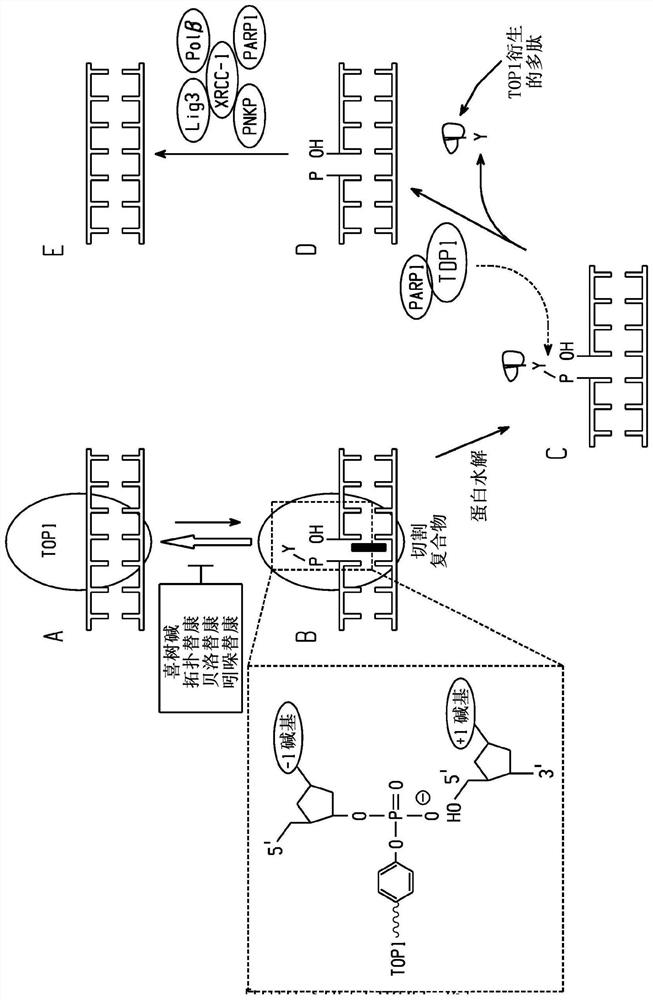
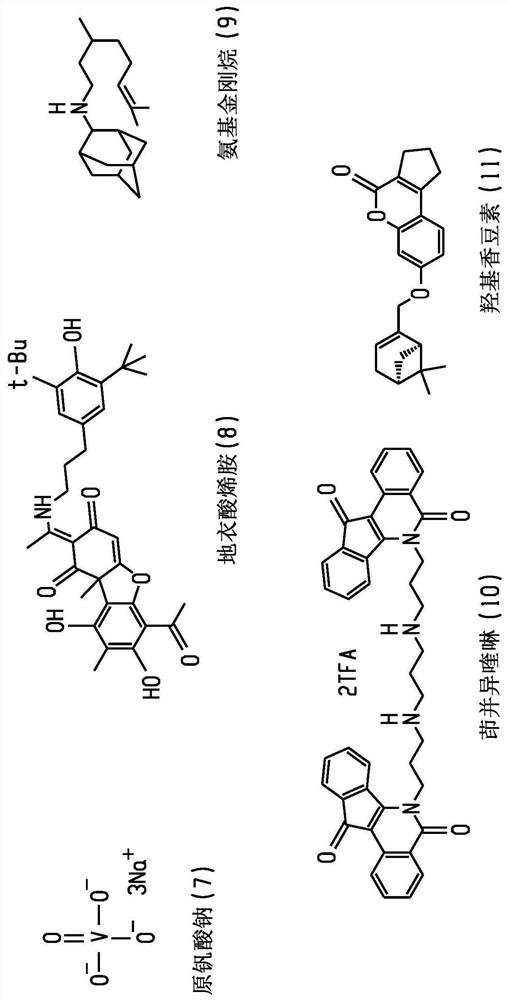
Britianone base derivatives useful as inhibitors of topoisomerase Ib (TOP1) and/or tyrosyl-DNA phosphodiesterase 1 (TDP1)
A C1-C6, C1-C2 technology for use as an inhibitor of topoisomerase IB (TOP1) and/or tyrosyl-DNA phosphodiesterase 1 (TDP1) derivatization of xanthoxyline realm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Example 1. General procedure for the synthesis of compounds
[0203] General procedure for the synthesis of Schiff bases 12a, 12b and 33. The reaction solution of 6-bromoveratrol (9.8 g, 40 mmol) and amine (ethanolamine, 3-aminopropanol or 4-methoxybenzylamine, 42 mmol) in methanol (200 mL) was stirred at room temperature for 12 h, then Concentrate under reduced pressure. The residue was washed with petroleum ether (2x10 mL) to give a white solid. 1 H NMR spectroscopy showed that the Schiff base intermediate was pure and was used in the next synthesis without further purification.
[0204]N-(2-hydroxyethyl)-6-bromoveratraldimine (12a). 1 H NMR (CDCl 3 )δ8.58(s, 1H), 7.54(s, 1H), 6.99(s, 1H), 3.95-3.89(m, 8H), 3.79(t, J=4.7Hz, 2H).
[0205] N-(3-hydroxypropyl)-6-bromoveratrol aldimine (12b). 1 H NMR (CDCl 3 )δ8.53(s, 1H), 7.45(s, 1H), 7.00(s, 1H), 3.91-3.86(m, 8H), 3.82(t, J=6.2Hz, 2H), 1.96(quint, J =6.0Hz, 2H).
[0206] N-(4-Methoxybenzyl)-6-bromoveratrol aldi...
Embodiment 2
[0252] Example 2. Synthesis of compounds 39–45
[0253] The 6-alkoxy substituted benzophenanthridone derivatives (39a / b-45a / b) were synthesized as shown in Scheme 2.
[0254]
[0255] The reagents and conditions used in Scheme 2 were as follows: (a) MeOH, room temperature. (b)i)N 2 , Compound 14, Ni(cod) 2 ,P(o-Tol) 3 , MeCN, 80℃; ii) CsOH, K 3 Fe(CN) 6 , MeOH, H 2 O, 80℃. (c)(COCl) 2 ,DMSO,TEA,DCM,-60℃. (d) HCl, AcOH, room temperature. (e) POCl 3 , DMF, 100℃. (f)HOCH 2 CH 2 R, THF, NaH, 70°C. (g)Br(CH 2 ) 3 Br, DMF, NaH, room temperature. (h) Amine (for dimethylamine, in a pressure vessel), DMF, K 2 CO 3 , KI, room temperature.
[0256] Synthesis of 2,3-Dimethoxy-[1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridine-13(12H)- Ketone (36). A reaction solution of 35 (270 mg, 0.5 mmol) and concentrated hydrochloric acid (0.4 mL) in acetic acid (4 mL) was added to a 50 mL round bottom flask. The flask was sealed with a rubber stopper. The reaction solution w...
Embodiment 3
[0288] Example 3: Synthesis of compounds 50-63 and 67
[0289] Scheme 3 outlines the synthesis of 5-aminoethyl substituted benzophenanthridone derivatives 50-63 and 67.
[0290]
[0291] The reagents and conditions used in Scheme 3 are as follows: (a) PBr 3 , TCM, room temperature; (b) Pd / C, H 2 (g), THF, room temperature; (c) Formaldehyde solution, Zn, CH 3 CO 2 H,H 2 O, room temperature; (d) RCl, DIPEA, DCM, room temperature.
[0292] Replacing the hydroxyl group of 17a with bromine afforded bromide 65 in 91% yield. After substituting the bromide with an azide group, a Pd / C catalytic reduction reaction was carried out under a hydrogen atmosphere to afford the target amine 67 in 51% yield from 65 (two steps). The primary amine intermediate 67 was reacted with formaldehyde to form a Schiff base, which was reduced using zinc dust to give intermediate 50 in 59% yield. 67 and 50 in dichloromethane with appropriate reagents (eg RC(O)Cl, RSO 2 Acylation of Cl, dialkyl ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com