Method for preparing phosphoric acid and byproduct fertilizer by using nitrophosphate fertilizer freezing process
A nitrophosphate fertilizer and process technology, applied in chemical instruments and methods, calcium/strontium/barium nitrate, phosphorus compounds, etc., can solve the problem of high cost, achieve the effect of increasing mineral components and facilitating absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
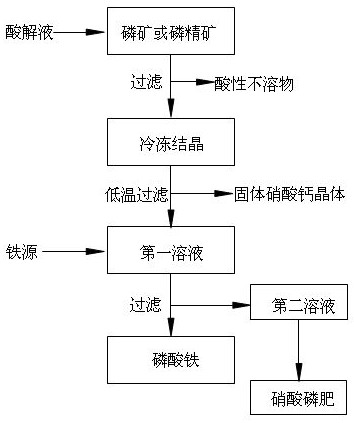
[0022] S2: The solution decomposed in S1 for refrigeration and crystallization. After the frozen crystals, low -temperature filtration is performed to remove the calcium nitrate crystals to prepare ammonium nitrate fertilizers. Obtaining the first solution, the amount of sulfuric acid is calculated according to the amount of Moore of the sulfuric acid root. The amount of Moore of the sulfuric acid root is 97%of the calcium ions in the filter liquid; The rate of ℃ / min is heated to 3 ° C and kept for 5min, then cooled at a rate of 1 ° C / min to -10 ° C, and keep it for 5 minutes.
[0023] S5: It is recycled to the second solution in S3 for preparation of phosphoric acid phosphoric fertilizer.
Embodiment 2
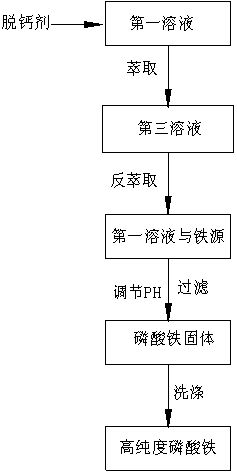
[0025] S3: In the case of stirring, add iron to the solution of the regulating pH obtained by S2, the nitrate iron is calculated by the molar amount, and the amount of molar amount of the nitrate iron is the amount of Moore in the solution in the solution in the solution of the pH 85%, and the stirring is filtered after the reaction is completed, the generated iron phosphate is removed, and the second solution is obtained;
[0026] In S3, before joining the iron source, first of all
[0027]After washing, the pure water was poured into the second solution and mixed in the second solution, and then nitrate fertilizer was prepared through the second solution.
Embodiment 3
Reference Figure 1-2 It is shown that the method of preparing phosphoric acid and by -yield fertilizer for phosphate fertilizer technology is used as the following steps:
S1: The phosphorus or phosphorus ore is decomposed through nitric acid to filter and remove acid insoluble objects;
S2: The decomposed solution in S1 is frozen and crystals, and then low-temperature filtration is performed after freezing crystals. Keep it for 5 minutes at 10 ° C, then heat up at a rate of 1 ° C / min to 3 ° C and keep it for 5min, then cool down at a rate of 1 ℃ / min to -10 ° C, keep it for 5 minutes, so that the circulation is 40min.
[0029] S3: In the case of stirring, add iron oxide (or tritenuxial two iron) to the first solution in S2. After adding tritenuxial dioxide, if the pH value of the solution is lower than 1, the pH value of the ammonia regulating solution is 1.5, and then the stirring will be filtered after the reaction is completed, the generated iron phosphate is removed, and the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com