Novel IL-15 fusion protein for resisting tumors
A fusion protein and a new type of technology, applied in the field of molecular biology, can solve the problems of systemic immune side effects, achieve the effects of improving anti-tumor efficacy, simplifying the production process, and increasing binding affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
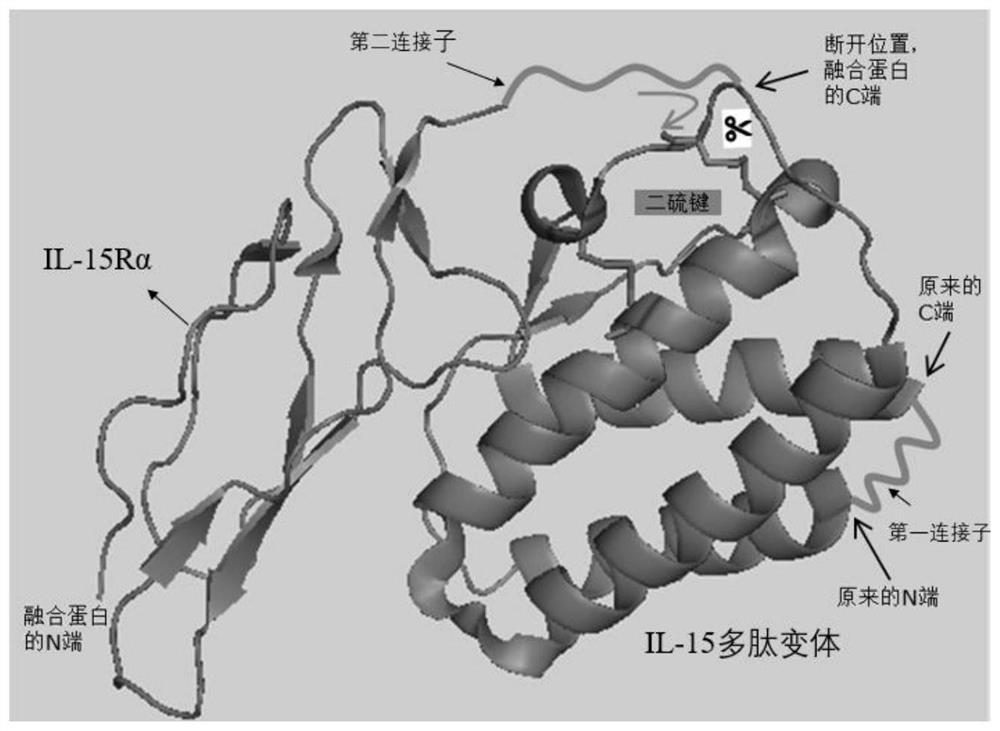
[0073] In the IL-15 M1 embodiment, the natural amino terminus and carboxyl terminus of IL-15 are linked with a first linker such as the sequence shown in SEQ ID NO. Cleavage occurs at the peptide bond, resulting in new amino and carboxy termini.
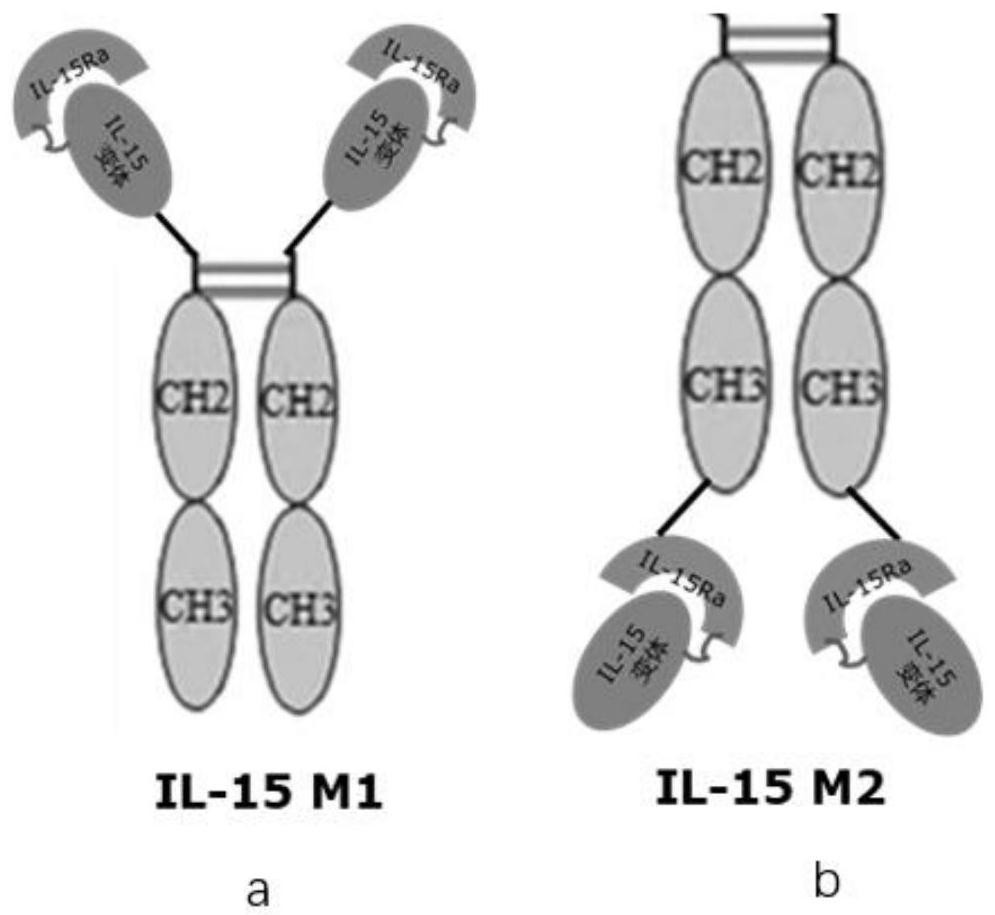
[0074] The IL-15Rα polypeptide is fused to the new amino terminus of the IL-15 polypeptide variant through a second linker, and then the new fusion protein is directly fused to the amino terminus of the hIgG1 Fc domain, such as figure 2 a shown. Those skilled in the art can design other IL-15 M1 variants satisfying the present invention, such as Fc domain-IL-15Rα polypeptide-second linker-IL-15 polypeptide variant, based on the teachings given in the present invention and the prior art. body.
Embodiment 2
[0076] In the IL-15 M2 embodiment, the natural amino terminus and carboxyl terminus of IL-15 are linked with a first linker such as the sequence shown in SEQ ID NO. Cleavage occurs at the peptide bond, resulting in new amino and carboxy termini.
[0077] The IL-15Rα polypeptide is directly fused to the new amino terminus of the IL-15 polypeptide variant, and then the new fusion protein is fused to the carboxyl group of the hIgG1 Fc domain through a second linker such as the sequence shown in SEQ ID NO.4 The sequence shown in SEQ ID NO.3 of the third linker is fused to the amino terminus of the Fc domain, as shown in figure 2 b shown.
Embodiment 3
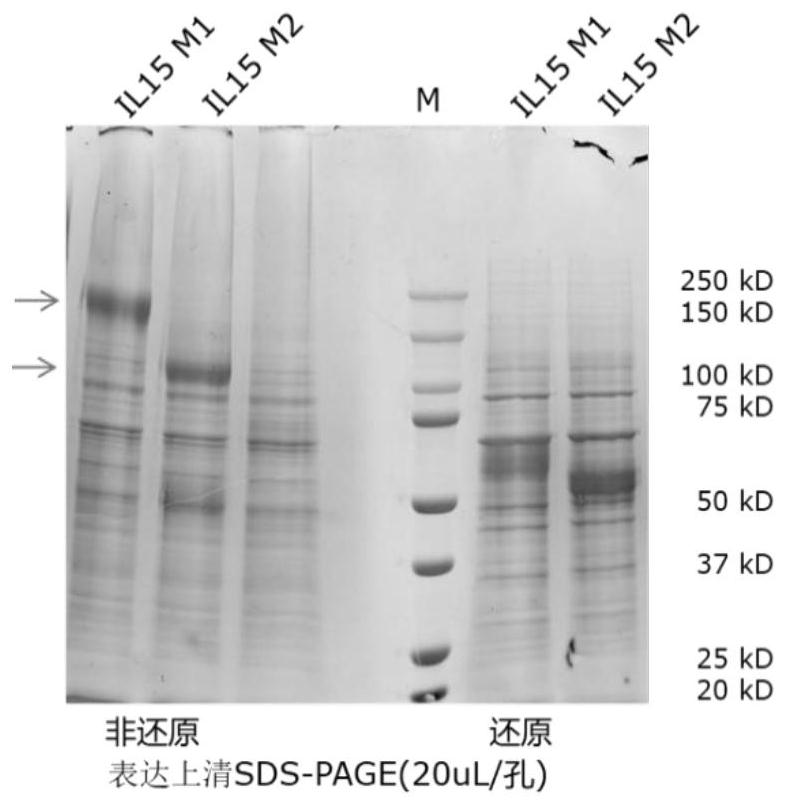
[0079] IL-15 M1 and IL-15 M2 were synthesized and expressed separately, and purified and prepared by means of molecular Fc domain tags. The breaking point is the peptide bond following the E82 residue. The expression host was mammalian cells (HEK293 or CHO).
[0080] i. Expression plasmid synthesis
[0081] Suzhou Jinweizhi Biotechnology Co., Ltd. was entrusted to synthesize the genes encoding IL-15 M1 and IL-15 M2, and then according to the operation method mentioned in the "Molecular Cloning Experiment Guide", DH10B was transformed, sequenced, and bacteria were preserved to obtain the corresponding plasmids.
[0082] ii. Plasmid extraction and HEK293 cell preparation
[0083] ii-1 plasmid extraction
[0084] According to the operation method mentioned in the "Molecular Cloning Experiment Guide", DH10B was transformed, sequenced, preserved and cultured. According to the operation methods mentioned in "Qiagen Mini-prep Kit" and "Qiagen Endofree Maxi-prep Kit", IL-15 M1 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com