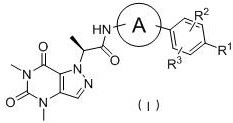
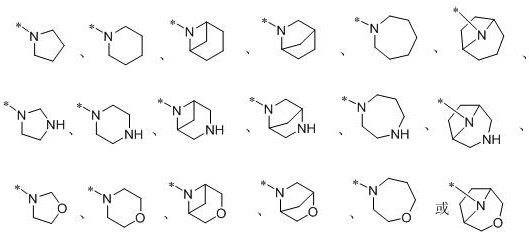
Pyrazolopyrimidine compound, isomer or salt as well as preparation method and application of pyrazolopyrimidine compound and isomer or salt
A technology for compounds and stereoisomers, applied in the field of medicinal chemistry, can solve problems such as weak activity, and achieve the effect of good antitussive effect and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
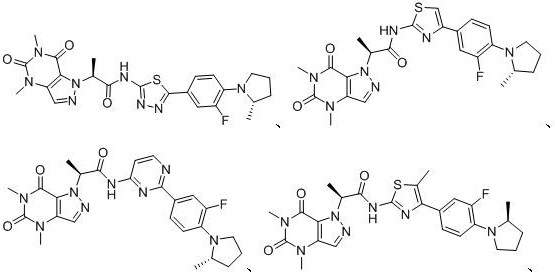
[0080] Example 1: (S)-2-(4,6-Dimethyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-D]pyrimidine- 1-yl)-N-(5-(3-fluoro-4-(S)-2-methylpyrrolidin-1-yl)phenyl)-1,3,4-thiadiazol-2-yl) Preparation of propionamide:
[0081]
[0082] Step 1: (S)- 2-(4,6-dimethyl-5,7-dioxo-4,5,6,7-tetrahydro-1h-pyrazolo[4,3-d]pyrimidine-1 - preparation of methyl) propionate
[0083]
[0084] Add 1-methyl-3,4,5,7-tetrahydro-1H-purine-2,6-dione (690mg, 4.15mmol) and K into a 25ml three-necked flask 2 CO 3 (0.573 g, 4.15 mmol), DMF (7 mL), stir and mix well. Methyl (R)-2-(methylsulfonyloxy)propionate (0.58 g, 3.2 mmol) was added and the reaction was stirred at room temperature overnight, the reaction was complete, then washed with saturated NH 4 Cl (20ml) quenched. The resulting mixture was extracted with EA (3 x 20 mL). The combined organic phases were washed with water (3 x 50 mL) and brine. Anhydrous Na for organic phase 2 SO 4 Dry and concentrated. The residue was separated and purifi...
Embodiment 2
[0107] Example 2: (S)-2-(4,6-Dimethyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-D]pyrimidine- Preparation of 1-yl)-N-(4-(3-fluoro-4-(S)-2-methylpyrrolidin-1-yl)phenyl)thiazol-2-yl)propionamide
[0108]
[0109] Step 1: Preparation of (S)-1-(3-fluoro-4-(2-methylpyrrolidin-1-yl)phenyl)ethane-1-one
[0110]
[0111] Add (S)-dimethylpyrrolidine (190mg, 2.23mmol), 3,4-difluoroacetophenone (317mg, 2.03mmol), potassium carbonate (309mg, 2.23mmol) to a 25ml reaction flask, heat to 80°C for reaction overnight. After the reaction was completed, the temperature was lowered to room temperature, water was added, extracted with EA, the layers were separated, dried and concentrated to dryness to obtain 400 mg of a yellow oily product with a yield of 88.9% and a purity of 98.16%.
[0112] ESI-MS: m / z=222.2(M+H) + .
[0113] Step 2: Preparation of (S)-2-bromo-1-(3-fluoro-4-(2-methylpyrrolidin-1-yl)phenyl)ethane-1-one
[0114]
[0115] A 25ml reaction flask was charged with (S)...
Embodiment 3
[0124]Example 3: (S)-2-(4,6-Dimethyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrazolo[4,3-D]pyrimidine- Preparation of 1-yl)-N-(2-(3-fluoro-4-(S)-2-methylpyrrolidin-1-yl)phenyl)pyrimidin-4-yl)propionamide
[0125]
[0126] Step 1: Preparation of (S)-3-fluoro-4-(2-methylpyrrolidin-1-yl)benzimidazole
[0127]
[0128] 3,4-Difluorobenzamide hydrochloride (308mg, 1.60mmol), (S)-dimethylpyrrolidine (150mg, 1.76mmol), K 2 CO 3 (664mg, 4.81mmol), DMSO (5ml), heated to 100°C with stirring for 5h. TLC monitored the reaction of the raw materials, cooled to room temperature, added water, extracted with EA, and the organic phase was concentrated to dryness. The concentrate was separated and purified by column chromatography (MeOH:DCM=2:100), the product was collected and concentrated to dryness to obtain 180 mg of the title product, yield 51%, and purity was 98.50%.
[0129] ESI-MS: m / z = 222.1(M+H) + .
[0130] Step 2: Preparation of (S)-2-(3-fluoro-4-(2-methylpyrrolidin-1-yl)phen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com