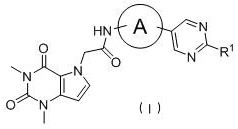
Pyrrolopyrimidine compound, isomer or salt, and preparation method and application thereof
A technology of compounds and stereoisomers, applied in the field of medicinal chemistry, can solve the problems of weak activity and achieve good antitussive effect and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
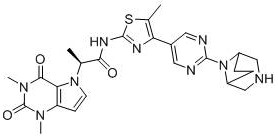
[0069] Example 1: (S)-2-(1,3-Dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrro[3,2-D]pyrimidine-5 -yl)-N-(5-(2-(S)-2-methylpyrrolidin-1-yl)pyrimidin-5-yl)-1,3,4-thiadiazol-2-yl)propanamide preparation
[0070]
[0071] Step 1: (S)-2-(1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrole[3,2-D]pyrimidine-5- Preparation of methyl) propionate
[0072]
[0073] Add 1,3-dimethyl-1,5-dihydro-2h-pyrrole[3,2-d]pyrimidine-2,4(3H)-dione (743.6mg, 4.15mmol) and K into a 25ml three-necked flask 2 CO 3 (0.622 mg, 4.5 mmol), DMF (8 mL), stirred and mixed. Add (R)-methyl 2-(methylsulfonyloxy)propanoate (0.58 g, 3.2 mmol). The reaction was stirred at room temperature overnight, the reaction was complete, then washed with saturated NH 4 Cl (20ml) quenched. The resulting mixture was extracted with EA (3 x 20 mL). The combined organic phases were washed with water (3 x 50 mL) and brine. Anhydrous Na for organic phase 2 SO 4 Dry and concentrated. The residue was separated and purifi...
Embodiment 2
[0092] Example 2: (S)-2-(1,3-Dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrro[3,2-D]pyrimidine-5 -yl)-N-(4-(2-(S)-2-methylpyrrolidin-1-yl)pyrimidin-5-yl)thiazol-2-yl)propanamide
[0093]
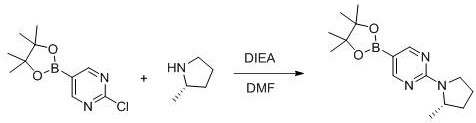
[0094] Step 1: Preparation of (S)-4-(2-(2-methylpyrrolidin-1-yl)pyrimidin-5-yl)thiazol-2-amine
[0095]
[0096] Add (S)-2-(2-methylpyrrolidin-1-yl)-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolato) to a 25ml three-necked flask Cyclopentan-2-yl)pyrimidine (200 mg, 0.69 mmol), 4-bromothiazol-2-amine (124 mg, 0.69 mmol), Pd (Ph 3 p) 4 (80mg, 0.069mmol), potassium carbonate (286mg, 2.07mmol), dioxane (3ml), water (0.6ml), N 2 After three replacements, it was heated to 1055°C for reaction for 6h. After the reaction was completed, EA was added, extracted and washed with water, the organic phase was dried and concentrated to dryness, and passed through a silica gel column to obtain 100 mg of a yellow oily product with a yield of 55.3%.
[0097] ESI-MS: m / z = 262.1(M+H) + .
[0098] Step 2:...
Embodiment 3
[0103] Example 3: (S)-2-(1,3-Dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrro[3,2-D]pyrimidine-5 -yl)-N-(5-methyl-4-(2-(S)-2-methylpyrrolidin-1-yl)pyrimidin-5-yl)thiazole-2-propionamide
[0104]
[0105] The preparation method is the same as the preparation method of Example 2, except that 4-bromothiazol-2-amine in step 1 is replaced with equimolar 4-bromo-5-methylthiazol-2-amine, and the method in step 2 is the same to obtain a yellow solid. The title compound, the two-step reaction yield: 36.2%, the purity is 98.42%.
[0106] ESI-MS: m / z =509.2(M+H) + .
[0107] 1 HNMR (400 MHz, DMSO-d6) δ: 12.75 (s, 1H), 8.56 (s, 2H), 8.03-8.10 (d, 1H), 6.23 (d, 1H), 5.88 (d, 1H), 4.30 ( s, 1H), 3.59 (m, 1H), 3.46 (s, 3H), 3.19 (s, 3H), 2.48 (s, 3H), 2.08 (s, 3H), 1.95 (s, 1H), 1.88 (d , 3H), 1.72 (s, 1H), 1.24 (d, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com