Proliposome testosterone undecanoate preparation
A technology of proliposome and testosterone undecanoate, which can be applied in the directions of liposome delivery, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as performance differences.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
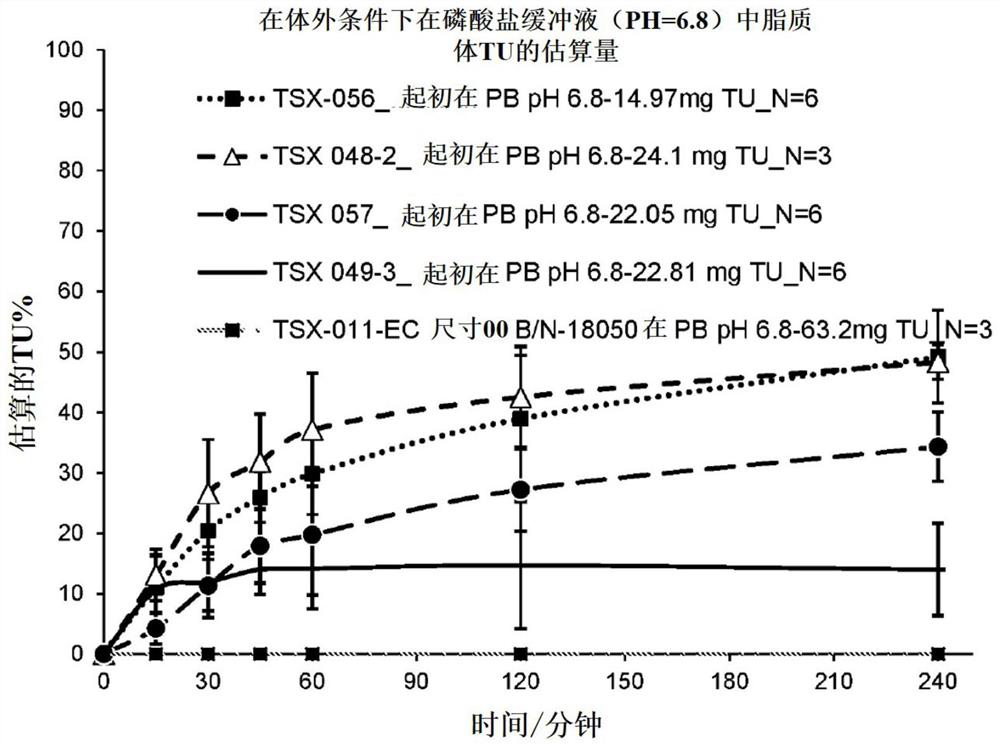
[0087] In vitro testing of TU proliposomal powder dispersion candidate formulations: TU release in phosphate buffer (pH 6.8). The percentage of TU released over time in phosphate buffer (pH 6.8) was determined for the following encapsulated TU proliposomal powder dispersion formulations: TSX-011; TSX-048; TSX:049 ; TSX-056; and TSX-057. For TSX-011 and TSX-048, n=3. n=6 for TSX-049, TSX-056 and TSX-057. The ingredients of each test formulation are described in Table 1. For solubility testing, one capsule was added, each to 250 mL of 0.2 M sodium triphosphate containing 1% w / v SLS. The final concentration of SLS in the composite medium was 0.25% w / v. The pH of the medium was adjusted to 6.8 using 2N HCl or 2N NaOH, and the dissolution conditions were maintained at 37±0.5°C for 4 hours at 75 RPM. Aliquots were collected at 0, 15, 30, 60, 90, 120 and 240 minutes and filtered using a 5μ glass fiber filter and then evaluated using high pressure liquid chromatography (HPLC). H...
Embodiment 2
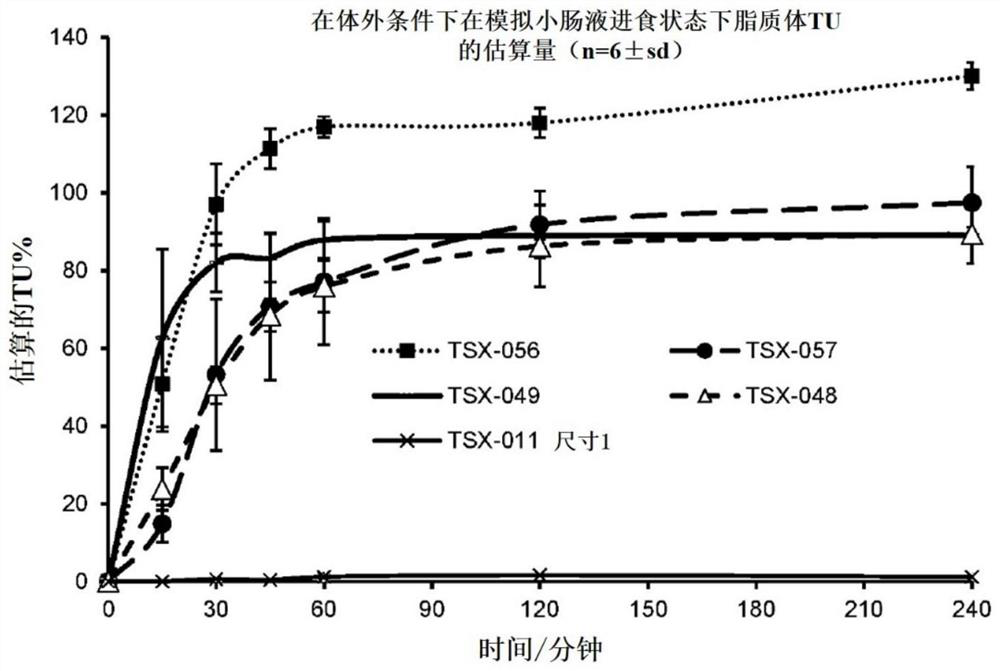
[0092] In vitro testing of TU proliposomal powder dispersion candidate formulations: TU release under simulated small intestinal fluid fed state. The percentage of TU released over time in 0.1N HCl solution was determined for the following encapsulated TU proliposomal powder dispersion formulations: TSX-011; TSX-048; TSX:049; TSX-056; and TSX-057. n=6 for each formulation. The ingredients of each test formulation are described in Table 1. The 0.1N HCl dissolution medium simulates the fed state in small intestinal fluid. The dissolution of each capsule was performed in 750 mL of 0.1 N HCl and maintained at 37 ± 0.5 °C for 4 hours at 75 RPM. Aliquots were collected at 0, 15, 30, 60, 90, 120 and 240 minutes and filtered using a 5μ glass fiber filter and then evaluated using HPLC as described in Example 1. Solubility data such as figure 2 reported.
Embodiment 3
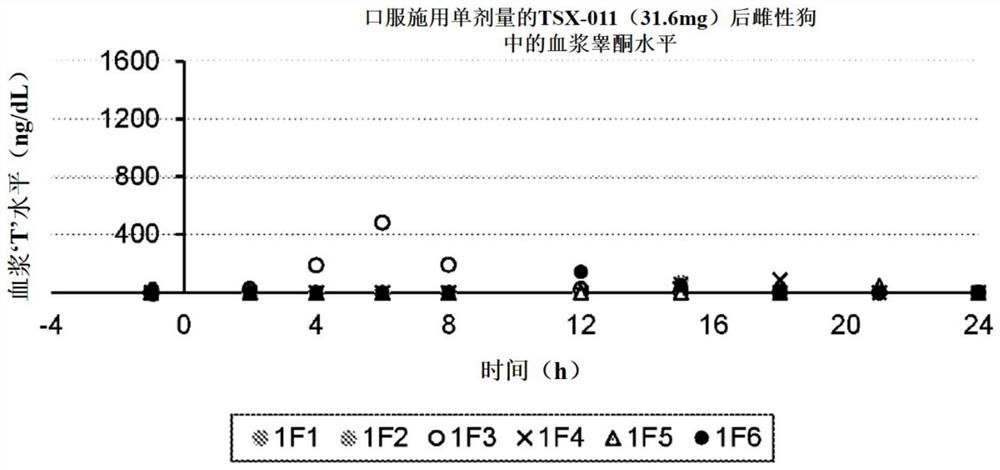
[0094] A single-dose study was designed to evaluate dose-response rates in female dogs. Dose response rates in dogs were assessed for TSX-011; TSX-048; TSX:049; TSX-056 and TSX-057. Each formulation was administered orally to female beagle dogs under fasting conditions. The ingredients of the application formulations are described in Table 1 . For the study, the dogs were fasted overnight and dosing (1 capsule / formulation / dog) was administered orally the next morning. The formulation dose for each formulation was normalized based on the body weight of each dog (mg of formulation administered / kg of body weight). See Table 2.
[0095] Table 2 Standardized formulation doses for dogs (administered per kg body weight)
[0096]
[0097] Blood samples were collected from each dog by jugular puncture 1 hour before oral administration, and then at 2, 4, 6, 8, 12, 15, 18, 21, and 24 hours after administration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com