Gabapentin impurity compound and preparation method thereof
A technology of gabapentin and compounds, which is applied in the field of gabapentin impurity compounds and its preparation, and can solve problems such as product quality impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
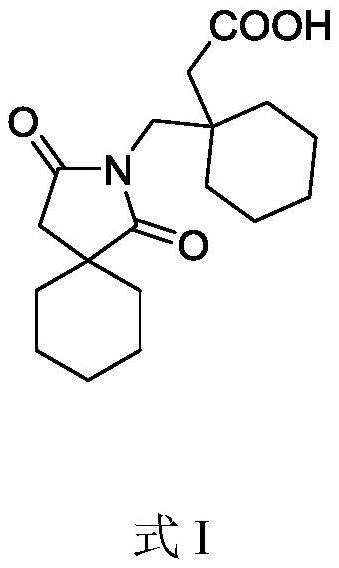
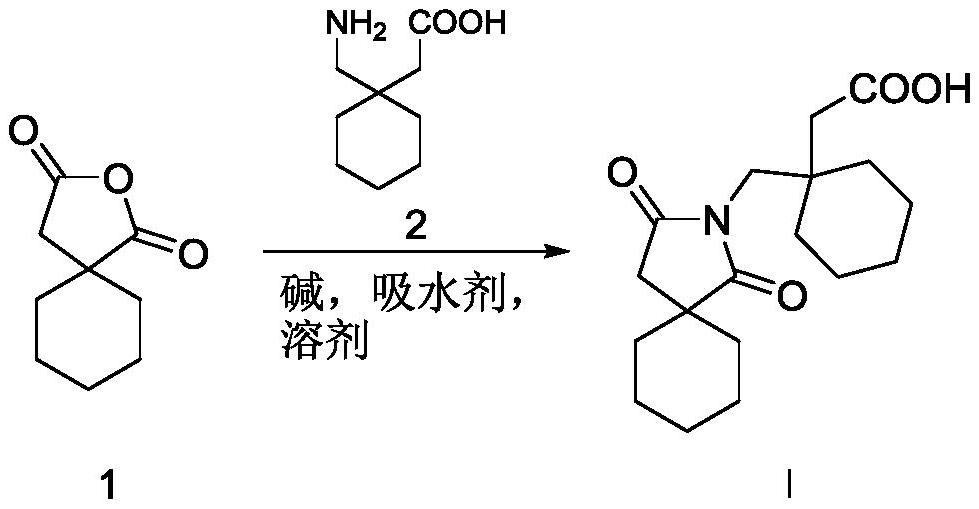
[0015] Example 1: 20.0 g of 2-oxaspiro[4.5]decane-1,3-dione (compound 1) was combined with 24.4 g of 2-(1-(aminomethyl)cyclohexyl)acetic acid (compound 1) 2) Add 300 mL of toluene, add 15.1 g of sodium carbonate and 10 g of sodium sulfate, and heat to 80° C. to react for 24 hours. After the reaction is completed, the system is cooled to room temperature 15~30°C, filtered to obtain a filtrate, the filtrate is decompressed to obtain a crude product, and 2-(1-((1,3-dioxa-2-aza is obtained through column chromatography. Spiro[4.5]decan-2-yl)methyl)cyclohexyl)acetic acid 25.2 g, yield 66.0%.
Embodiment 2
[0016] Example 2: Combine 20.0 g of 2-oxaspiro[4.5]decane-1,3-dione (compound 1) with 28.4 g of 2-(1-(aminomethyl)cyclohexyl)acetic acid (compound 2) Add 300 mL of 1,4-dioxane, add 19.6 g of potassium carbonate and 10 g of magnesium sulfate, and heat to 90° C. to react for 24 hours. After the reaction is completed, the system is cooled to room temperature 15~30°C, filtered to obtain a filtrate, the filtrate is decompressed to obtain a crude product, and 2-(1-((1,3-dioxa-2-aza is obtained through column chromatography. Spiro[4.5]decan-2-yl)methyl)cyclohexyl)acetic acid 27.1 g, yield 71.0%.
Embodiment 3
[0017] Example 3: Combine 20.0 g of 2-oxaspiro[4.5]decane-1,3-dione (compound 1) with 40.7 g of 2-(1-(aminomethyl)cyclohexyl)acetic acid (compound 2) Add 300 mL of dimethyl sulfoxide, add 14.3 g of triethylamine and 10 g of calcium chloride, and heat to 100° C. to react for 24 hours. After the reaction is completed, the system is cooled to room temperature 15~30°C, filtered to obtain a filtrate, the filtrate is decompressed to obtain a crude product, and 2-(1-((1,3-dioxa-2-aza is obtained through column chromatography. Spiro[4.5]decan-2-yl)methyl)cyclohexyl)acetic acid 21 g, yield 55.0%.
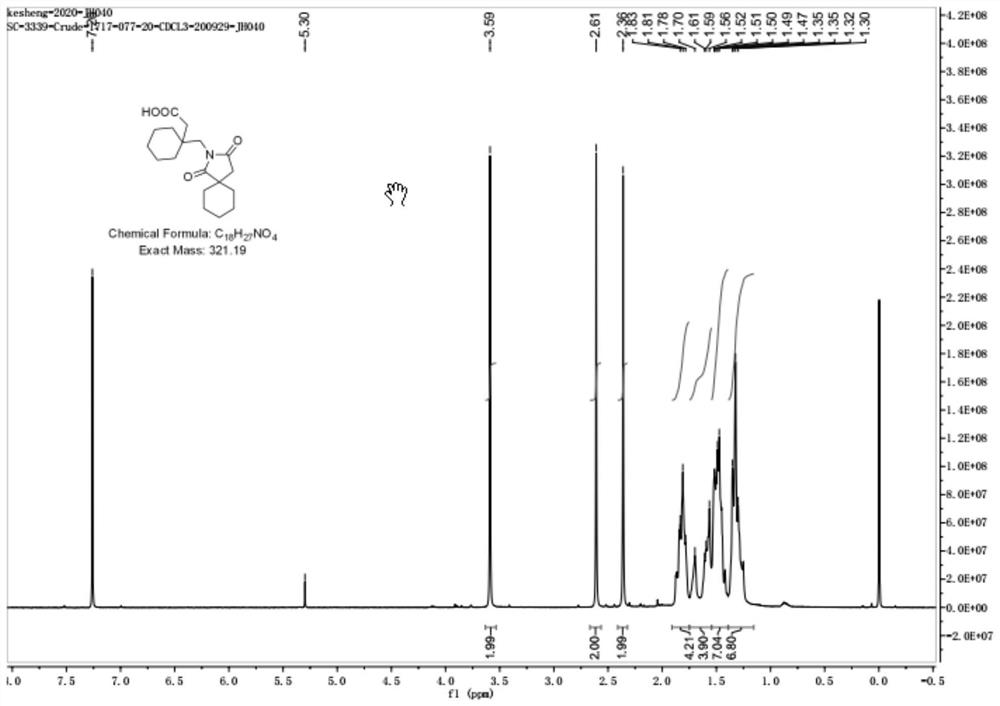
[0018] 1 H-NMR (d 6 -CDCl3)δ(ppm): 3.59(2H,s), 2.61(2H,s), 2.36(2H,s), 1.78-1.83(4H,m), 1.56-1.61(4H,m), 1.30-1.52 (13H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com