Quantitative detection method for creatine and creatinine in whole blood
A quantitative detection method and technology of creatine, applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by chemical reaction of materials, etc., can solve the deviation of test results, different sample sizes, complex processing, etc. problem, to achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
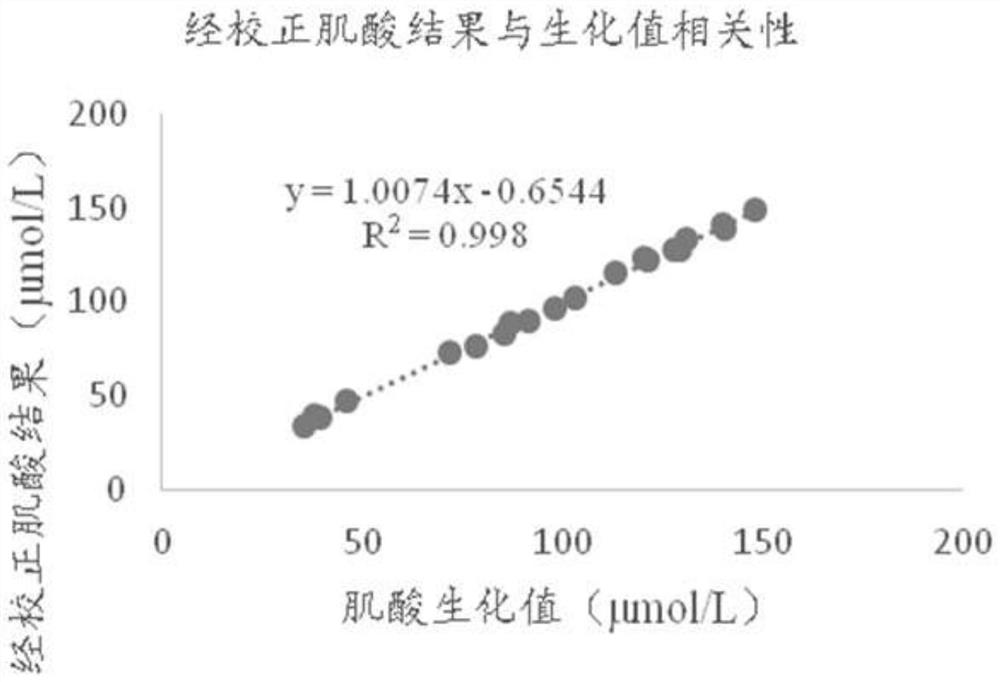
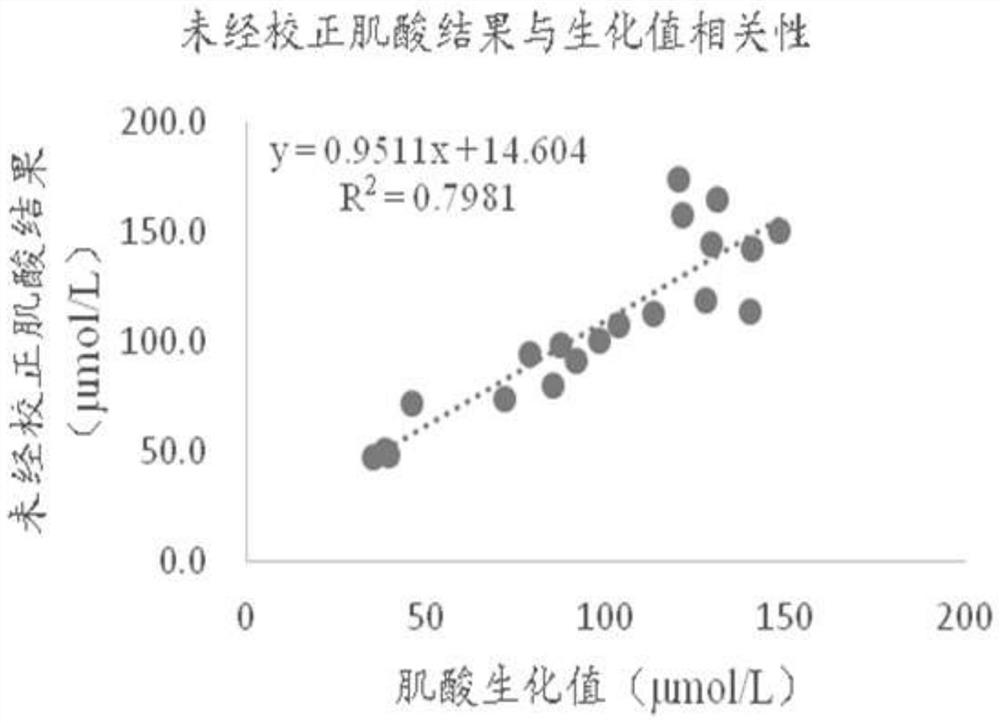
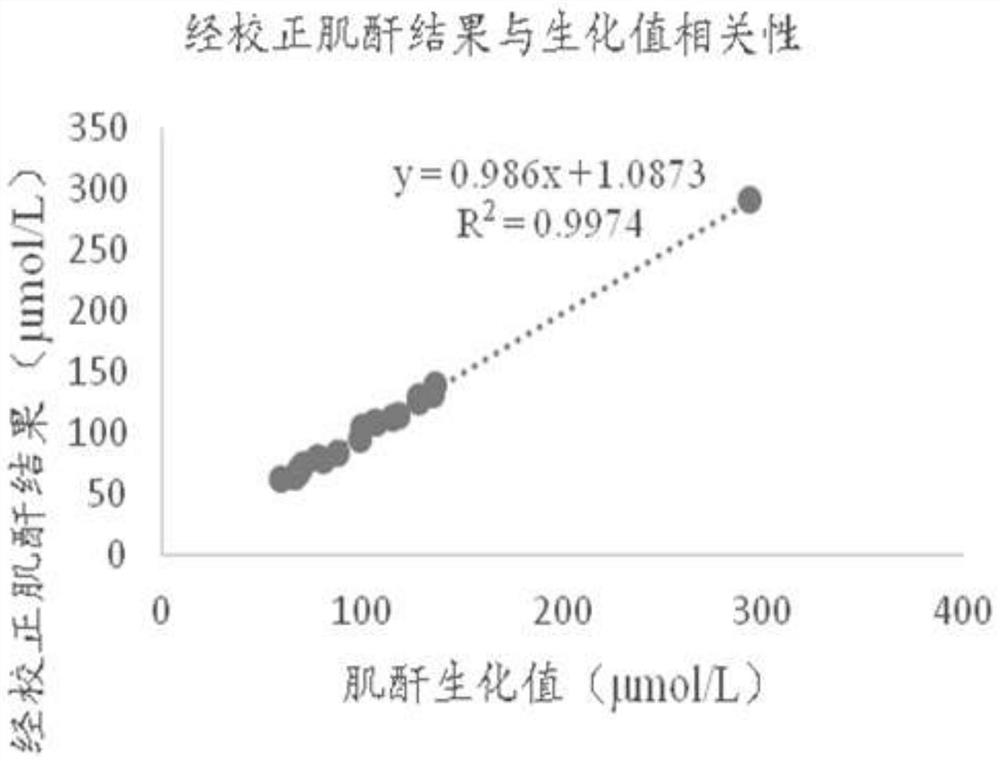
[0055] The dry chemical test strip for combined creatine-creatinine detection used in this example includes a lower plate of the jam, a reaction membrane, a blood filter membrane, a diffusion membrane, and an upper plate of the jam. The upper plate of the cartridge is provided with a sample adding port, a first detection window, a second detection window and a third detection window. The first detection window corresponds to the first detection area, the second detection window corresponds to the second detection area, and the third detection window corresponds to the third detection area.
[0056] The whole blood sample is added from the sample injection port, and the sample will quickly and evenly diffuse through the diffusion membrane and seep down. In the first detection area, the hemoglobin reacts with the substance on the reaction membrane to produce a color change, and different colors will produce different degrees of light. Reflection, the signal receiver of the dry b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com