Compositions for colon cleansing and treatment of gastrointestinal disorders
A pharmacy, allylglycine technology, applied in the direction of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve the problems of low adenoma detection, long operation time, short examination interval and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
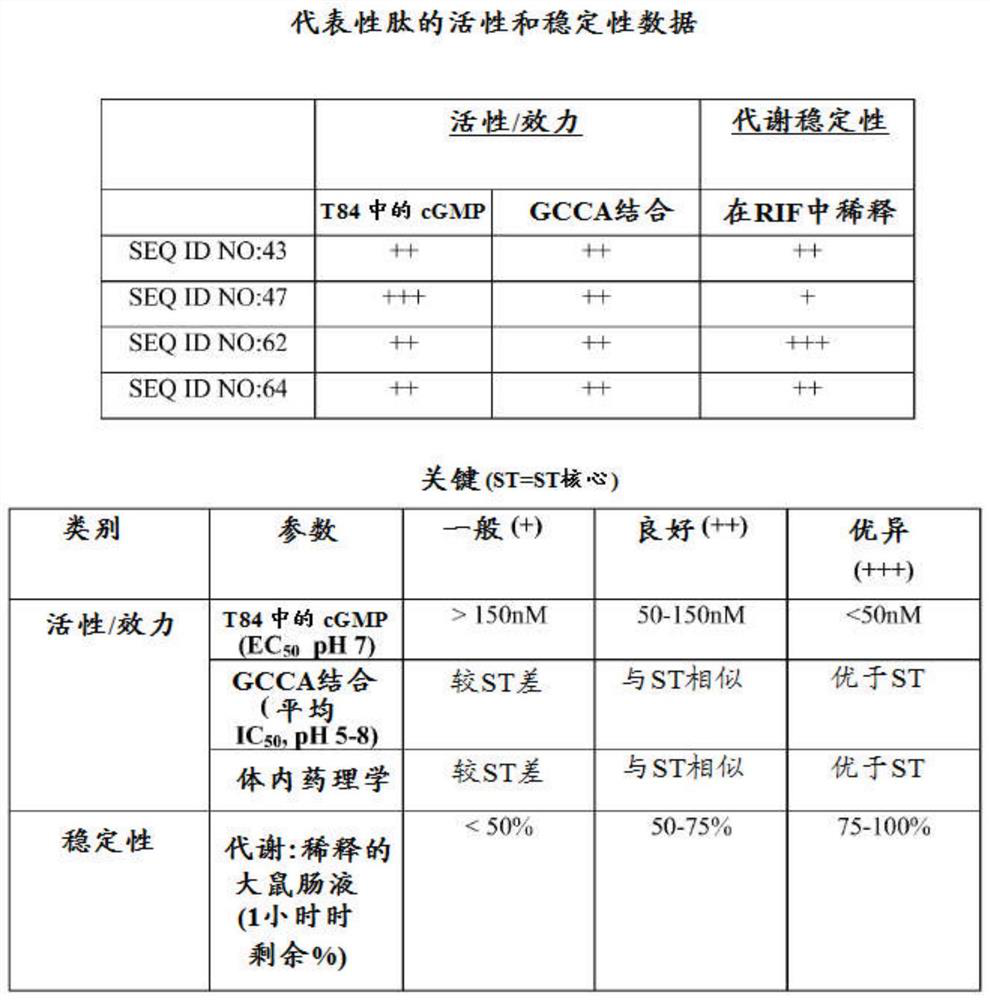
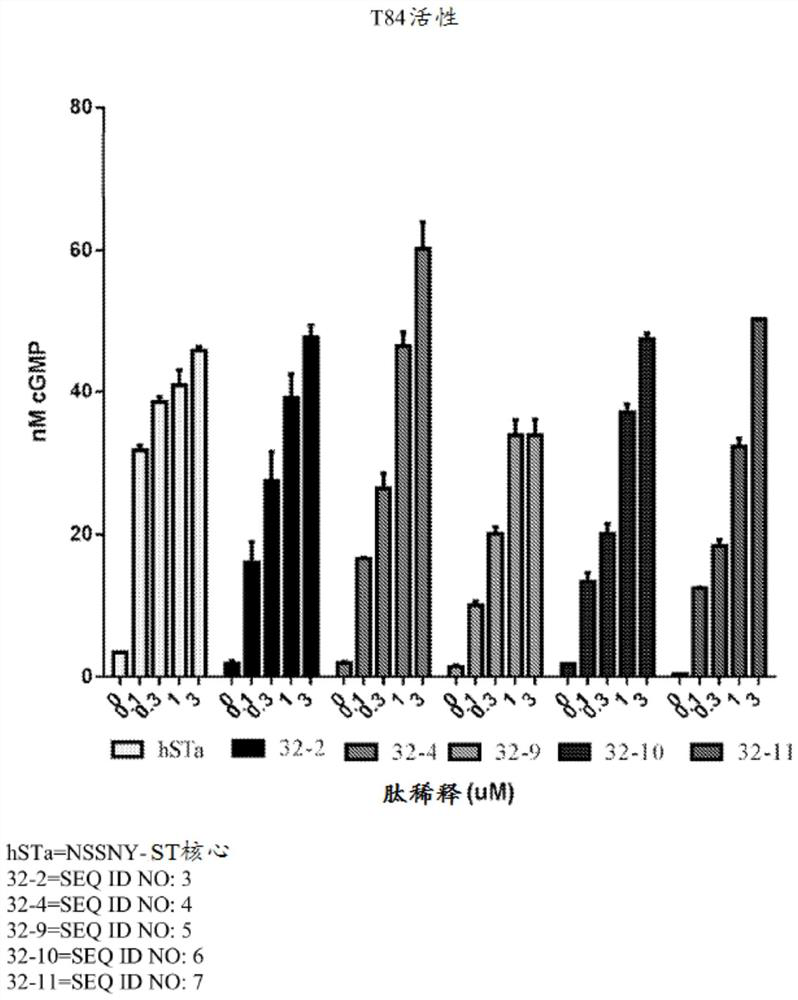
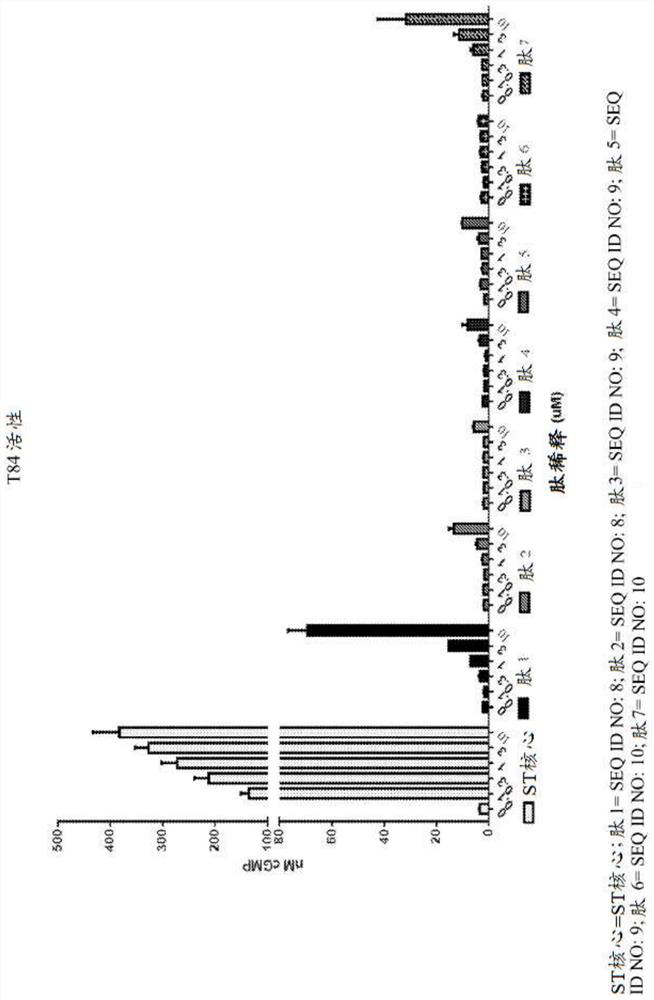
[0589] Example 1: cGMP accumulation in T84 cells for analysis of GC-C activity
[0590] For cGMP assays, 2.0x10 5 Cells / mL of T84 cells were grown overnight in 96-well tissue culture plates. The next day, T84 cells were washed twice with 200 μL DMEM+20 mM MES (pH 5) or DMEM+50 mM sodium bicarbonate (pH 8) or DMEM pH 7 without additives. These buffers do not contain serum. After the second wash, cells were incubated with 180 μL of 1 mM isobutylmethylxanthine (IBMX) in buffer pH 5, 7 or 8 for 10 minutes at 37° C. to inhibit any phosphodiesterase activity. The peptides were then diluted to 10-fold concentration in pH 5, 7 or 8 buffer. Dilute 20 μL of the peptide solution to a final volume of 200 μL with T84 cells to make each peptide concentration 1×. Eleven point curve analysis was performed for each peptide and final peptide concentrations in nM were tested in each assay: 10000, 3000, 1000, 300, 100, 30, 10, 3, 1, 0.3, 0.1.
[0591] There is no peptide control used to de...
Embodiment 2
[0596] Example 2: GC-C binding assay
[0597] All GC-C bindings were performed in a final volume of 200 μL of media pH 5, 7 or 8. Medium was prepared at pH 5 using DMEM and 0.5% BSA and 20 mM 2-(N-morpholino)ethanesulfonic acid (MES). Medium was prepared at pH 7 using DMEM and 0.5% BSA. Medium was prepared at pH 8 using DMEM and 0.5% BSA containing 20 mM sodium bicarbonate.
[0598] T84 cells were used at a rate of 250,000 cells per reaction. Cells were grown to confluence on T-150 flasks using DMEM-F12 50 / 50 medium with 5 mM L-glutamine and 5% FBS. Cells were scraped using DMEM and 0.5% BSA and counted to determine how much volume to add per reaction to give 250,000 cells in a final volume of 200 μL. Reactions were then started at 200,000 CPM per reaction of I125-STp, cold peptide competitor, and T84 cells in sequence. The samples were then incubated at 37°C for 1 hour. After 1 hour, all samples were added to pre-blocked GF-C plates and aspirated through. Each well w...
Embodiment 3
[0599] Example 3: In vitro metabolic incubation of rat intestinal fluid (RIF)
[0600] Rat intestinal fluid was obtained by adding PBS to ligated rat jejunal loops for 30 minutes. Intestinal fluid was then collected, pooled and kept on ice before centrifugation at 4°C. The supernatant was removed and snap frozen. 60 [mu]M peptide (100 [mu]g / mL) was then added to rat intestinal fluid along with PBS and 0.5% BSA. Control incubations were performed in PBS. 50 μL aliquots were then taken in duplicate from all samples at 0, 10, 30 and 60 minutes and stopped with 12% trichloroacetic acid containing internal standard.
[0601] The samples were spun and the supernatant removed for analysis by LC-MS using the calculated exact mass of each peptide (or predicted metabolite) to generate an extracted ion chromatogram. The relative response factor (analyte peak area / internal standard peak area) for each sample was used to construct the percent remaining relative to time=0. Results fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com