Application of anisodine hydrobromide in treatment of Parkinson's disease
A technology of anisodine hydrobromide and hydrobromic acid, applied in the field of anisodine hydrobromide in the treatment of Parkinson's disease, to achieve the effect of improving motor function, improving quality of life, and preventing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
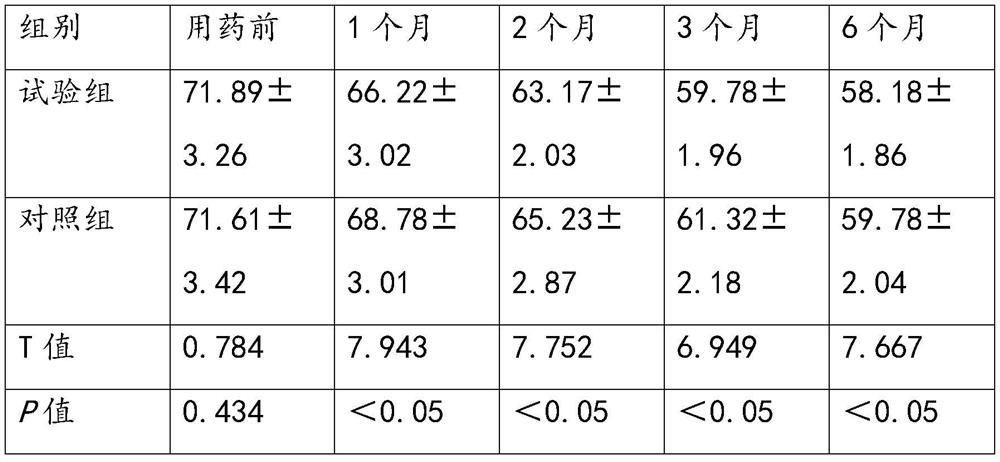
[0031] Embodiment 1. Clinical efficacy observation of anisodine hydrobromide tablets in the treatment of Parkinson's disease
[0032] 1.1. Experiment preparation
[0033] A prospective randomized controlled clinical trial method was used to select 300 patients who were diagnosed with Parkinson's disease in the research unit from March 2020 to March 2021, and who met the diagnostic criteria for Parkinson's disease in China (2016 version) and inclusion and exclusion criteria. , including 163 males and 137 females. According to the random number table, they were randomly divided into the experimental group and the control group, with 150 cases in each group. The mean age of the patients in the experimental group was 58.79±3.31 years, and the mean disease duration was 3.46±1.75 years; the mean age of the patients in the control group was 59.45±3.02 years, and the mean disease duration was 3.38±1.64 years. There was no significant difference in age and course of disease between t...
Embodiment 2
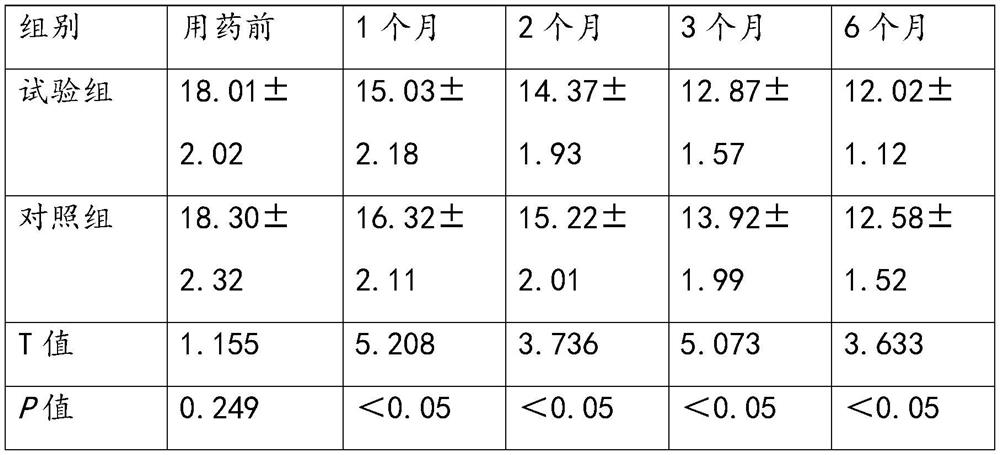
[0083] Embodiment 2. Clinical observation of the clinical efficacy of anisodine hydrobromide injection in the treatment of acute tremor in Parkinson's disease
[0084] 1.1. Experiment preparation
[0085] In this experiment, a prospective randomized controlled clinical trial method was used to select patients diagnosed with acute tremor of Parkinson's disease in the research unit from July 2020 to November 2021, who met the diagnostic criteria for Parkinson's disease in China (2016 version) and inclusion and exclusion criteria 280 patients, including 150 males and 130 females. According to the random number table, they were randomly divided into the experimental group and the control group, with 140 cases in each group. The mean age of the patients in the experimental group was 62.23±2.31 years, and the mean disease duration was 3.82±1.67 years; the mean age of the patients in the control group was 62.16±2.07 years, and the mean disease duration was 3.61±1.78 years. There wa...
Embodiment 3
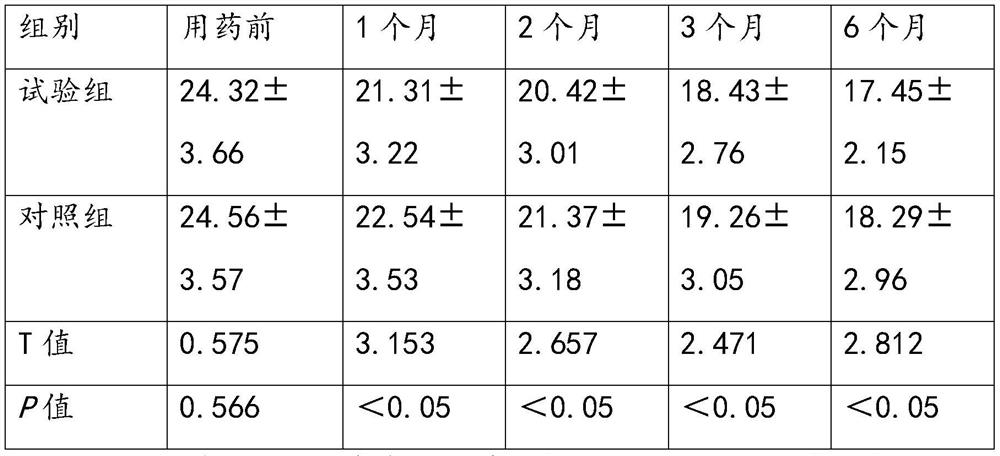
[0122] Embodiment 3. Observation on the clinical efficacy of sequential treatment of Parkinson's disease with Anisodine Hydrobromide Injection and Anisodine Hydrobromide Tablets
[0123] 1.1. Experiment preparation
[0124] In this experiment, a prospective randomized controlled clinical trial method was used to select patients diagnosed with Parkinson's disease in the research unit from December 2020 to December 2021, who met the diagnostic criteria for Parkinson's disease in China (2016 version) and the inclusion and exclusion criteria. 350 cases, including 195 males and 155 females. According to the random number table, they were randomly divided into the experimental group and the control group, with 175 cases in each group. The mean age of the patients in the experimental group was 58.43±3.48 years, and the mean disease duration was 3.37±1.72 years; the mean age of the patients in the control group was 58.24±3.14 years, and the mean disease duration was 3.52±1.61 years. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com