Method for preparing deuterated amino-acid esters by photocatalysis
A technology for the preparation of catalysis and amino acids, which is applied in organic chemical methods, cyanide reaction preparation, chemical instruments and methods, etc. It can solve the problems of uncontrollable sites, high cost and energy consumption, and restrict development, and achieve short reaction time and environmental protection. Good, the effect of reducing the cost of deuterium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
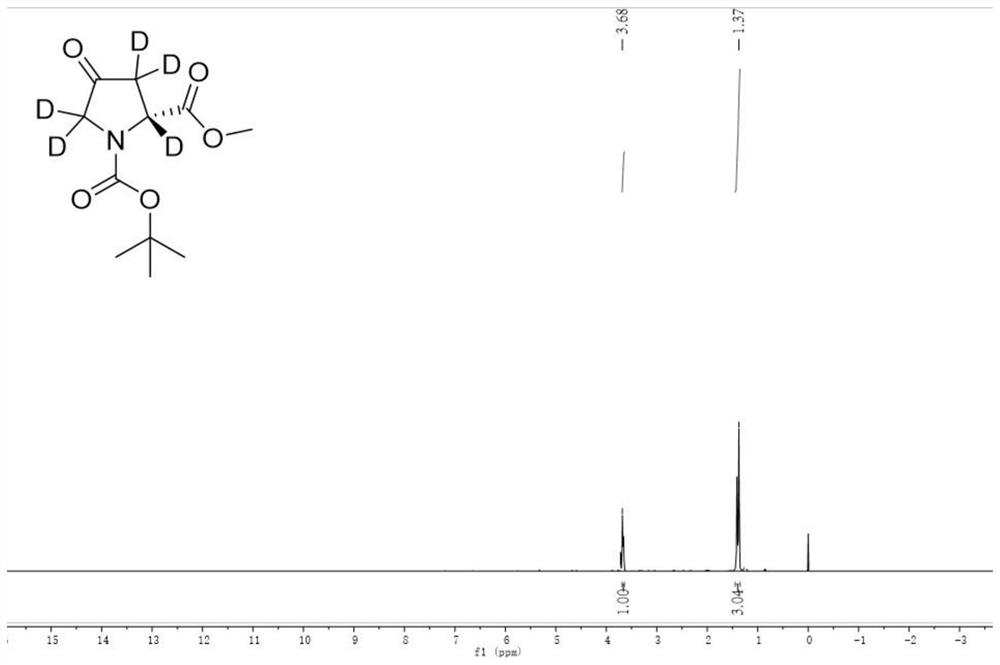
Image
Examples
Embodiment 1
[0043]
[0044] (1) Dissolve 500 mmol of the above-mentioned amino acid ester compound in 25 mol of deuterium water, and add a composite catalyst (ferric chloride: 0.025 mmol, tetraphenyl phosphine chloride: 0.025 mmol, sodium tetraphenyl boron: 0.5 mmol, Na 2 B 12 H 12 : 0.5mmol, Al 2 O 3 : 0.25 mmol), N 2 Stir well under protection;
[0045] (2) Under the irradiation of a UV lamp with a power of 5W and a wavelength of 365nm, at room temperature (25°C) for a full reaction for 8h;
[0046] (3) after the reaction finishes, remove the catalyst by filtration, extract with ethyl acetate / water=1:1, and get the organic phase;
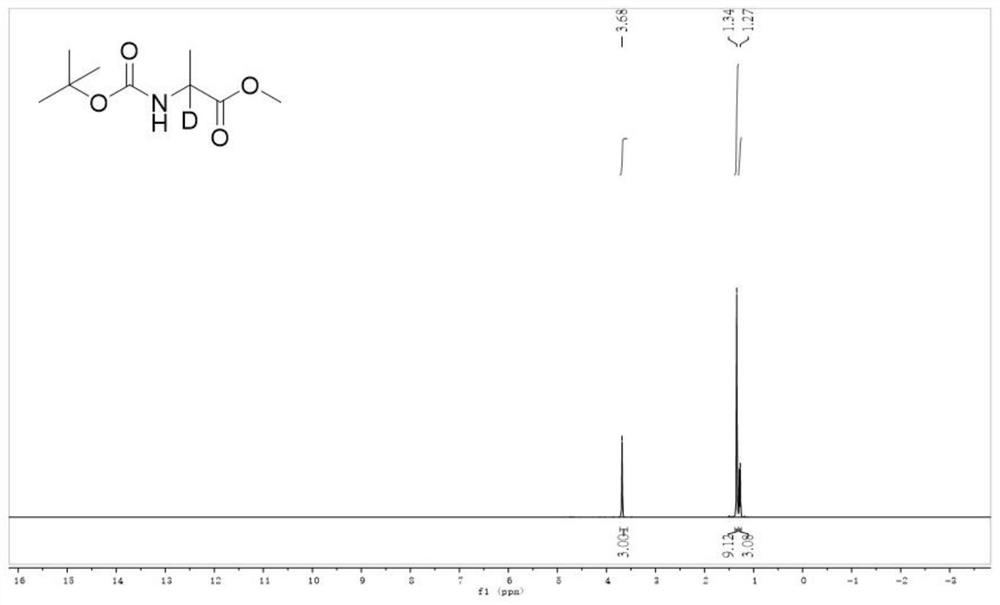
[0047] (4) The deuterated amino acid ester compound of the following formula can be obtained by separation by column chromatography. The isolated yield was 91% according to 1 The H NMR spectrum calculated the deuterium incorporation of this compound to be 97%.
[0048] 1 H NMR (400 MHz, D6-DMSO): δ 3.68 (m, 3H), 1.34 (m, 9H), 1.29-1.27 (m, 3H). ...
Embodiment 2
[0050]
[0051] (1) Dissolve 10 mmol of the above amino acid ester compound in 500 mmol of deuterium water, and add a composite catalyst (HCl: 0.1 mmol, tetraphenylphosphonium chloride: 0.1 mmol, potassium tetraphenylboron: 0.1 mmol, Cs 2 B 10 H 10 : 0.05mmol, Al 2 O 3 : 0.1 mmol), N 2 Stir well under protection;
[0052] (2) Under the irradiation of a UV lamp with a power of 25W and a wavelength of 265nm, fully react for 4h at room temperature (25°C);
[0053] (3) after the reaction finishes, the catalyst is removed by filtration, extracted with butyl acetate / water=1:1, and the organic phase is taken;
[0054] (4) The deuterated amino acid ester compound of the following formula can be obtained by separation by column chromatography. The isolated yield was 88% according to 1 The H NMR spectrum calculated the deuterium incorporation of this compound to be 95%.
[0055] 1 H NMR (400 MHz, D6-DMSO): δ 3.63 (m, 3H), 2.06-1.93 (m, 1H), 1.3 (m, 9H), 0.88-0.85 (m, 6H). ...
Embodiment 3
[0057]
[0058] (1) Dissolve 1 mol of the above-mentioned amino acid ester compound in 100 mol of deuterium water, and add a composite catalyst (AgNO 3 : 0.05mmol, Tetraphenylphosphonium chloride: 0.05mmol, Sodium tetraphenylboron: 0.5mmol, C 2 B 3 H 7 : 0.05mmol, Al 2 O 3 : 0.5 mmol), N 2 Stir well under protection;
[0059] (2) Under the irradiation of a UV lamp with a power of 50W and a wavelength of 395nm, fully react for 24h at room temperature (30°C);
[0060] (3) after the reaction finishes, the catalyst is removed by filtration, extracted with butyl acetate / water=1:1, and the organic phase is taken;
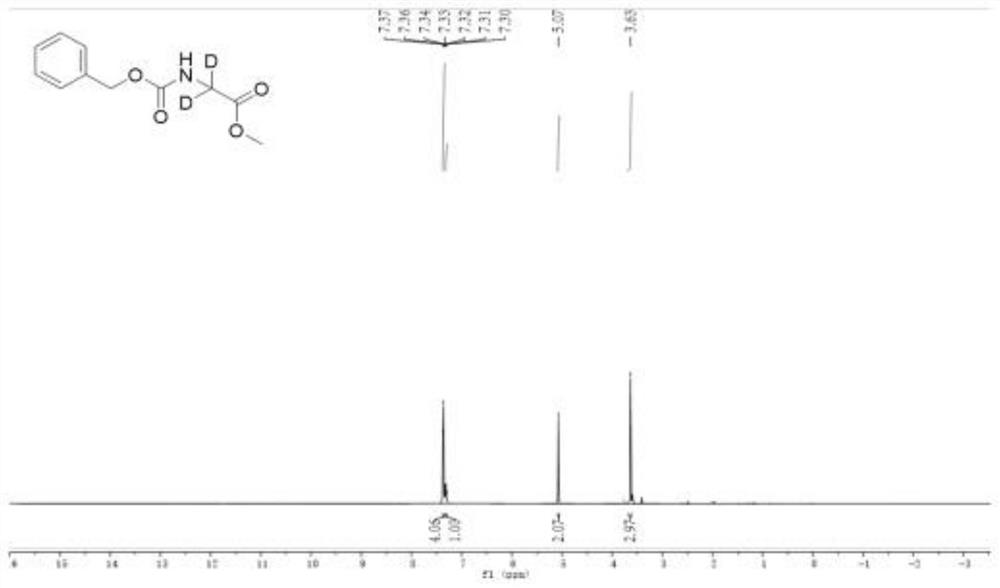
[0061] (4) The deuterated amino acid ester compound of the following formula can be obtained by separation by column chromatography. The isolated yield was 89% according to 1 The deuterium incorporation of this compound was calculated to be 96% from the H NMR spectrum
[0062] : 1 H NMR (400MHz, D6-DMSO): δ 7.37-7.36 (m, 2H), 7.34-7.32 (m, 2H), 7.31-7.30 (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com