Method for preparing deuterated acetonitrile through photoelectric integrated catalysis
A technology for the preparation of deuterated acetonitrile and catalysis, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds. Low generation cost, short response time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Add 10 mmol of acetonitrile to 100 mmol of deuterium water, and add a composite catalyst (deuterated DMSO: 5 mmol, H 2 SO 4 : 0.1 mmol, TiO 2 : 0.1 mmol, tetrabutylammonium tetrafluoroborate: 0.5 mmol, NaB 6 H 7 : 0.1 mmol), N 2 Stir well under protection;
[0029](2) The power is 20W, the wavelength is 365nm UV lamp irradiation, C(+) / Pt(-) is used as the electrode, and the current is 10mA, and the reaction is fully reacted at 25℃ for 0.5h;
[0030] (3) After the reaction, the catalyst was collected by filtration, and the filtrate was distilled under reduced pressure at 40°C to obtain a crude deuterated acetonitrile;
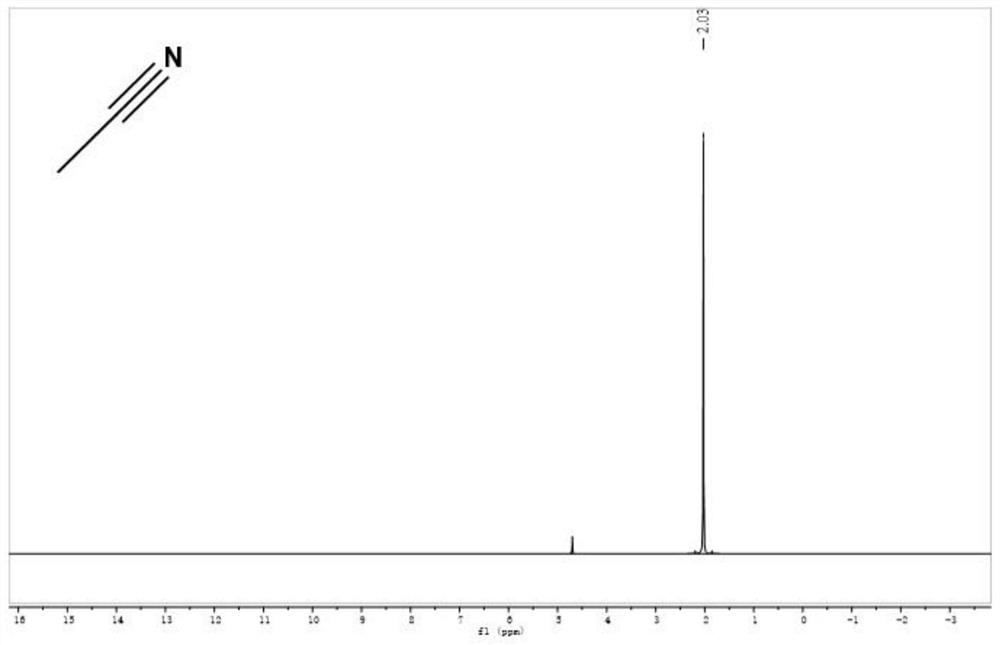
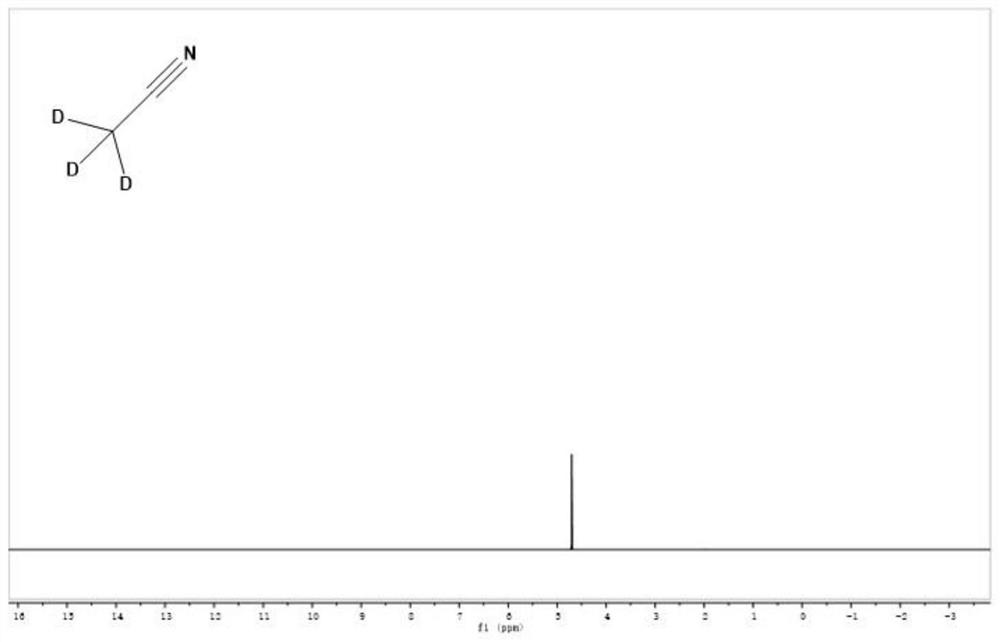
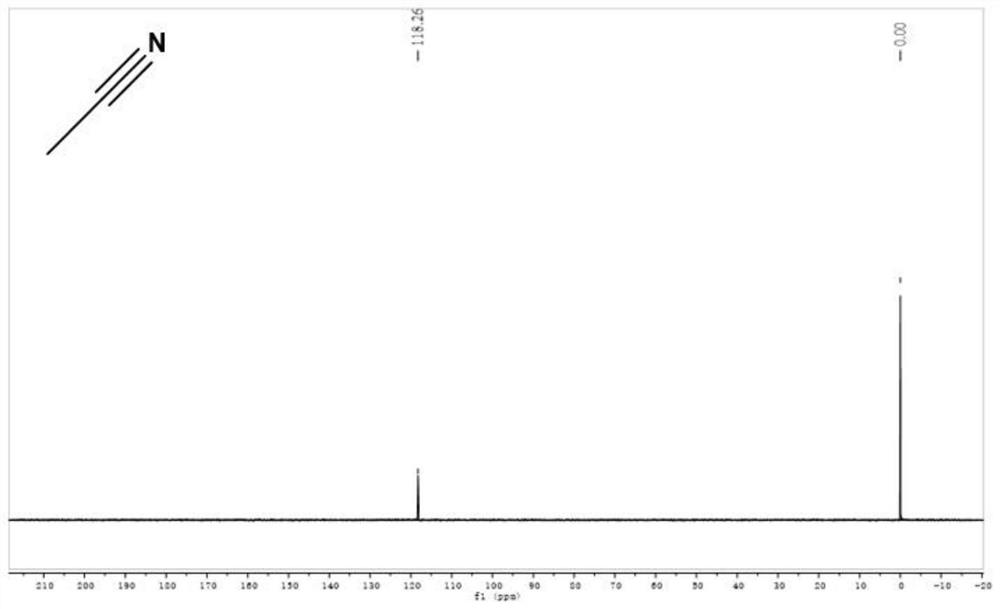
[0031] (4) Using anhydrous Na 2 SO 4 After drying for 24 h, pure deuterated acetonitrile can be obtained with a yield of 99.10%, and its deuteration rate detected by high-resolution mass spectrometry is 99.92%. The nuclear magnetic contrast spectra before and after deuteration of acetonitrile are shown in the accompanying drawings ( figure 1 ...
Embodiment 2
[0033] (1) Add 1 mmol of acetonitrile to 5 mmol of deuterium water, and add a composite catalyst (deuterated DMSO: 0.08 mmol, FeCl 3 : 0.02 mmol, TiO 2 : 0.05 mmol, tetramethylammonium chloride: 0.03 mmol, Cs 2 B 8 H 8 : 0.025 mmol, B 10 H 14 : 0.025 mmol), N 2 Stir well under protection;
[0034] (2) The power is 5W, and the wavelength is 390nm UV lamp irradiation, C(+) / Pt(-) is used as the electrode, and the current is 15mA, and the reaction is fully reacted at 20℃ for 1 h;
[0035] (3) After the reaction, the catalyst was collected by filtration, and the filtrate was distilled under reduced pressure at 40°C to obtain a crude deuterated acetonitrile;
[0036] (4) Using anhydrous MgSO 4 After drying for 24 h, pure deuterated acetonitrile can be obtained with a yield of 99.21%, and its deuteration rate detected by high-resolution mass spectrometry is 99.86%.
Embodiment 3
[0038] (1) Add 5 mmol of acetonitrile to 100 mmol of deuterium water, and add a composite catalyst (deuterated DMSO: 0.25 mmol, AgNO 3 : 0.025mmol, TiO 2 : 0.05mmol, tetrabutylammonium hexafluorophosphate: 0.025mmol, C 2 B 10 H 12 : 0.05 mmol), N 2 Stir well under protection;
[0039] (2) The power is 25W, and the wavelength is 265nm under the irradiation of ultraviolet lamp, C(+) / Pt(-) is used as the electrode, and the current is 20mA, and the reaction is fully reacted at 15℃ for 2 hours;
[0040] (3) After the reaction, the catalyst was collected by filtration, and the filtrate was distilled under reduced pressure at 40°C to obtain a crude deuterated acetonitrile;
[0041] (4) Using anhydrous CaCl 2 After drying for 24 h, pure deuterated acetonitrile can be obtained with a yield of 99.43%, and its deuteration rate detected by high-resolution mass spectrometry is 99.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com