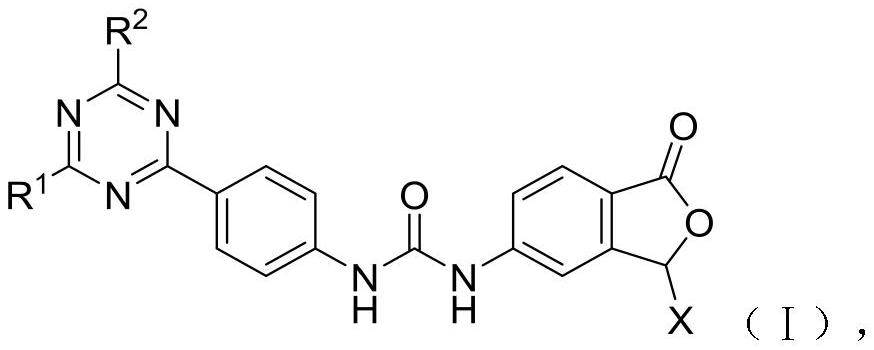
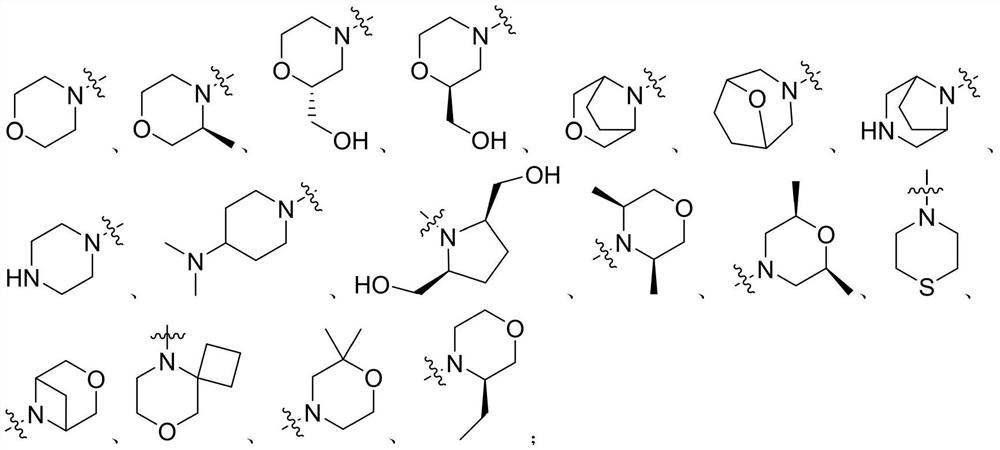
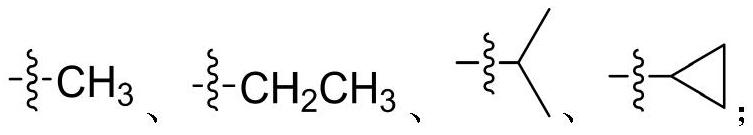
Diaryl urea mTOR kinase inhibitor as well as pharmaceutical composition and application thereof
The technology of a kinase inhibitor and diaryl urea, which is applied in the field of medicine, can solve the problems of non-target side effects and achieve the effects of low toxicity, cost reduction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: (S)-1-(4-(4-(3-Methylmorpholino)-6-morpholino-1,3,5-triazin-2-yl)phenyl)-3 -(1-oxo-1,3-dihydroisobenzofuran-5-yl)urea, the structural formula is as follows:
[0107]
[0108] The synthetic method includes the following steps:
[0109] Step 1: Synthesis of 4-(4,6-Dichloro-1,3,5-triazin-2-yl)morpholine
[0110] The structural formula of 4-(4,6-dichloro-1,3,5-triazin-2-yl)morpholine:
[0111]Weigh the raw material cyanuric chloride (10.84mmol) and put it into a 100mL double-necked flask, add DCM to dissolve, then add DIPEA (10.84mmol), and cool down at a low temperature of -78°C for 10 minutes after vacuum nitrogen circulation for three times. Morpholine (10.84 mmol) was slowly added to the liquid funnel and the reaction was kept at low temperature for 1 h. TLC monitoring was completed. The reaction solution was directly concentrated in vacuo and mixed with silica gel, and the obtained product was purified by silica gel column chromatography (PE:EA=5:1). ...
Embodiment 2
[0120] Example 2: (R)-1-(4-(4-(2-(hydroxymethyl)morpholino)-6-morpholino-1,3,5-triazin-2-yl)phenyl )-3-(1-oxo-1,3-dihydroisobenzofuran-5-yl)urea, the structural formula is as follows:
[0121]
[0122] Synthesis method: The substituted heterocyclic fragment in step 2 in Example 1 was changed to (R)-2-hydroxymethylmorpholine, and other steps and operations were the same as those in Example 1; white solid, yield: 17%. NMR data are 1 H NMR(600MHz,DMSO-d6)δ9.33(s,1H),9.17(s,1H),8.34-8.28(m,2H),7.90(s,1H),7.76(d,J=8.4Hz, 1H), 7.58(s, 2H), 7.52(d, J=9.3Hz, 1H), 5.36(s, 2H), 4.83(s, 2H), 4.77-4.43(m, 2H), 3.90(d, J = 51.7Hz, 5H), 3.67(s, 4H), 3.47(d, J=58.4Hz, 4H), 3.01(s, 1H), 2.76(s, 1H); 13 C NMR(151MHz,DMSO-d6)δ170.8,169.4,168.9,164.9,164.7,163.8,152.4,149.7,145.6,142.8,130.9,129.5,126.3,119.3,118.4,117.9,111.0,76.0,73.8. ,56.5,55.3.HRMS(ESI)calcd.for C 27 H 29 N 7 O 6 [M+H] + :548.2258,found:548.2253.
Embodiment 3
[0123] Example 3: 1-(4-(4-(4-(Dimethylamino)piperidin-1-yl)-6-morpholino-1,3,5-triazin-2-yl)phenyl )-3-(1-oxo-1,3-dihydroisobenzofuran-5-yl)urea, the structural formula is as follows:
[0124]
[0125] Synthetic method: The substituted heterocyclic fragment in step 2 in Example 1 was changed to 4-dimethylaminopiperidine, and other steps and operations were the same as those in Example 1. White solid, yield: 27%. NMR data are 1 H NMR(600MHz,DMSO-d6)δ9.35(s,1H),9.19(s,1H),8.28(d,J=7.2Hz,2H),7.90(s,1H),7.75(s,1H) ,7.57(s,2H),7.51(s,1H),5.35(s,2H),4.76(d,J=126.1Hz,2H),3.79(s,4H),3.65(s,4H),2.91( s, 2H), 2.37(ddq, J=11.5, 7.8, 3.7Hz, 1H), 2.18(s, 6H), 1.81(s, 2H), 1.30(d, J=11.3Hz, 2H); 13C NMR( 151MHz,DMSO-d6)δ170.8,169.4,165.2,164.6,152.4,149.7,145.6,142.8,131.1,129.5,126.3,119.3,118.4,118.0,111.0,70.0,66.5,62.02,43.7,42. HRMS(ESI)calcd.for C 29 H 34 N 8 O 4 [M+H] + :559.2781,found:559.2776.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com