Aspergillus sydowii MNP-2 and application of aspergillus sydowii MNP-2 in synthesis of dibenzoxane compounds
A technology of MNP-2 and Aspergillus polymosa, which is applied to medical preparations containing active ingredients, methods based on microorganisms, fungi, etc., can solve the problems of no compound synthesis method, and achieve the effect of improving activity and simple separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
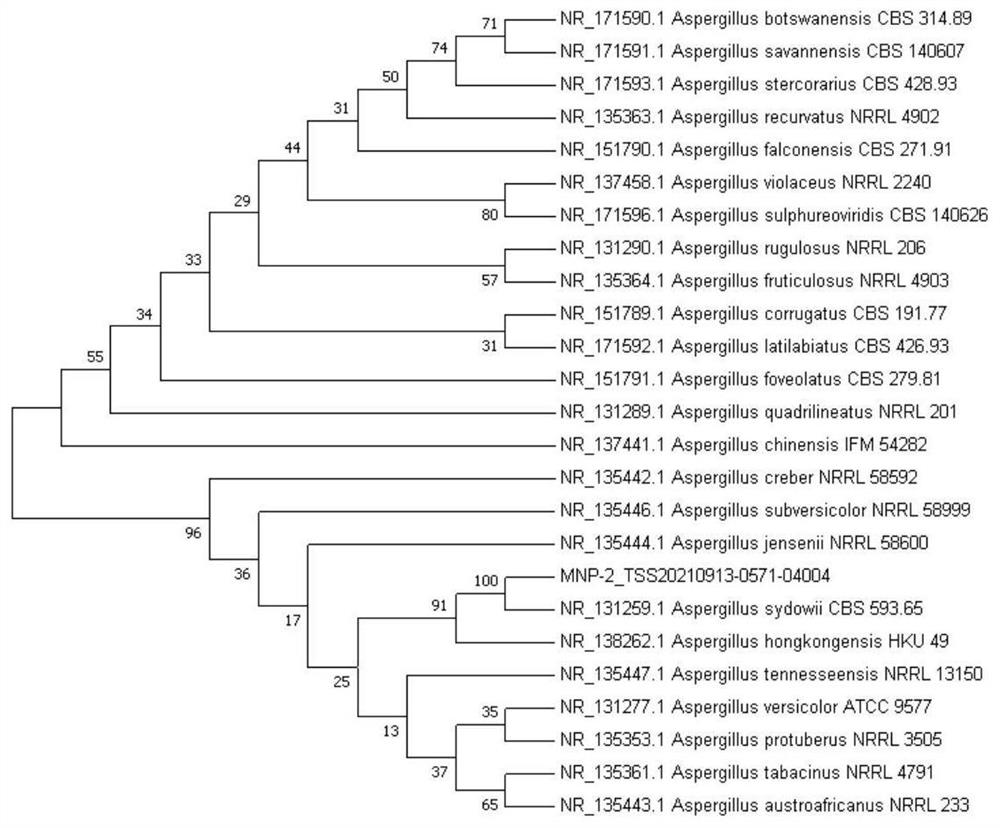
[0022] Example 1. Screening and identification of strain MNP-2
[0023] 1. Screening of strain MNP-2
[0024] Primary screening: 200 g of ore collected from the North Pole (longitude 73.8, latitude -168.9) was placed in a 15 mL centrifuge tube, 5 mL of sterile water was added, and sonicated at room temperature and 40 KHz for 5 min. After standing for stratification, the supernatant was collected. Put them into PDA plates, cultivate them in a 28°C incubator for 3-4 days, and select single colonies after the cells grow out. The final concentration composition of PDA plate medium is: glucose 20g / L, potato 200g / L, agar 18g / L, the solvent is distilled water, and the pH is natural.
[0025] Re-screening: inoculate the single colony from the primary screening into the PDA plate, incubate at 28°C for 3-4 days, and repeat the screening until the size, shape and color of the colony are basically the same, and finally the strain MNP-2 is obtained.
[0026] 2. Identification of strains ...
Embodiment 2
[0034] Example 2: Fermentation culture of strain MNP-2
[0035] (1) Activation culture: The strain MNP-2 was inserted into the PDA slant medium, and cultivated in a 28°C incubator for 3-4 days to obtain activated strains; the final concentration of the PDA slant medium was as follows: glucose 20g / L, potato 200g / L L, agar 18g / L, distilled water as solvent, natural pH.
[0036] (2) Seed cultivation: pick an inoculated loop from the activated colony in step (1) and inoculate it into the PDB seed medium, and shake it for 3 days at 200 rpm and 28°C to obtain seed liquid; the PDB seed medium is finally The concentration composition is: glucose 20g / L, potato 200g / L, the solvent is distilled water, and the pH is natural.
[0037] (3) Fermentation culture: connect 0.83L of the seed liquid obtained in step (2) to the surface of the 16.6kg rice medium with an inoculum of 0.05mL / g volume concentration, stir evenly, and leave it to ferment for 30 days in the dark at room temperature. day...
Embodiment 3
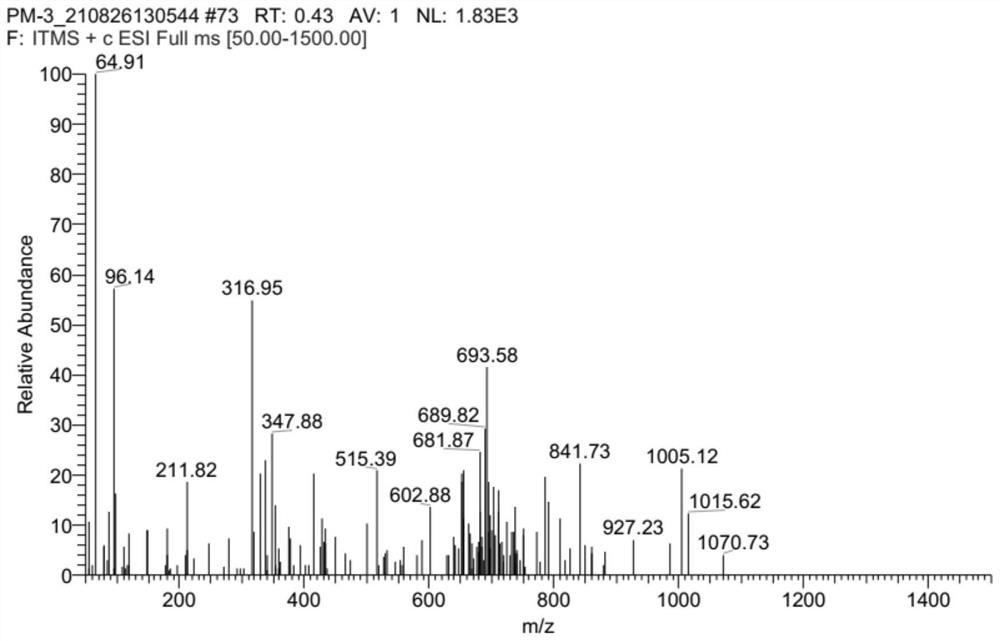
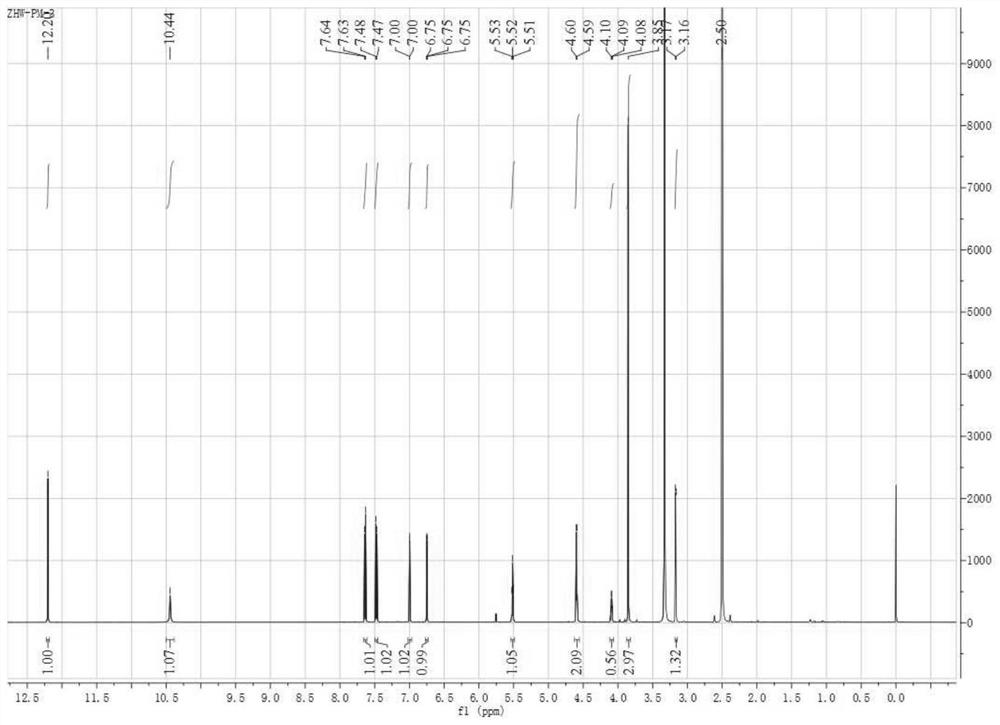
[0038] Example 3: Dibenzo[b,e]oxa Extraction, separation and identification of compound (I)
[0039] 1. Extraction and separation of compound (I)
[0040] The whole fermentation mixture prepared in Example 2 was added to 30L of ethyl acetate (the amount of ethyl acetate was calculated as 1.8ml / g based on the quality of the rice medium before fermentation), the room temperature, 40kHz ultrasonic extraction for 20min, filtered, and the filtrate was concentrated under reduced pressure to No liquid flows out to obtain crude extract extract; after dissolving all crude extract extract with 500mL of methanol, silica gel column (silica gel particle size 200-300 mesh, column height 120cm, diameter 10cm, column height 50cm) is used to carry out For separation and purification, the dichloromethane:methanol with volume ratios of 1:0, 100:1, 50:1, 20:1, 10:1, 4:1, and 0:1 was used as the eluent, and the elution rates were 60mL / min, the elution volume is 10 column volumes, and the efflue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com