Process for preparation of substituted nicotinamides
A technology of dimethylamino and methoxymethyl, applied in the field of preparation of substituted nicotinamide, which can solve the problems of cumbersome steps and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
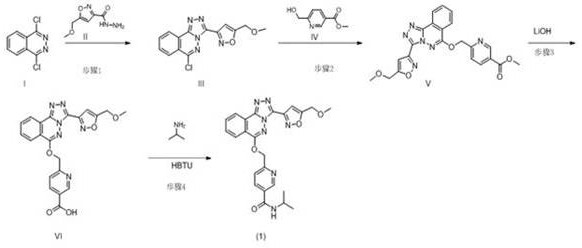
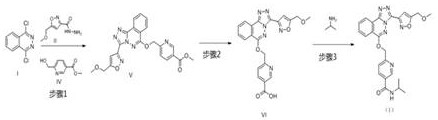
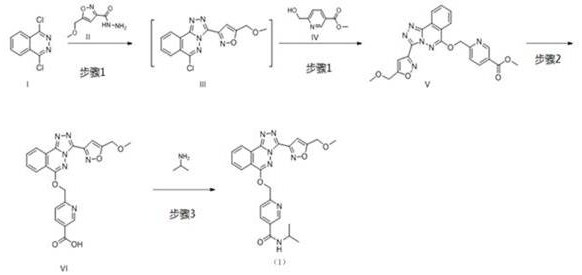
[0031] step 1:
[0032]At room temperature, add 31.73kg of acetonitrile in the reactor, add 2.00kg 1,4-dichlorophthalazine I and 1.81kg 5-(methoxymethyl)isoxazole-3-carbonyl hydrazide II under stirring, stir The temperature was raised to reflux (80°C) and reacted for 2 hours. The system was cooled to below 40 ℃, 31.73 kg of acetonitrile, 1.77 kg of 6-(hydroxymethyl) nicotinic acid methyl ester IV were added, stirred for 15-30 minutes, 8.53 kg of potassium phosphate was added, the temperature was raised to 45-55 ℃ and stirred for 20-24 hours, add water, stir, filter, and place the filter cake in an oven at 50-60 °C to dry to obtain 3.85 kg of 6-((((3-(5-(methoxymethyl)isoxazol-3-yl) -[1,2,4]Triazolo[3,4-a]oxazin-6-yl)oxy)methylnicotinate methyl V, yield 85.9%. 1H NMR (400 MHz, CHLOROFORM-d )δppm 3.39 (s, 3 H) 3.89(s, 3H), 4.71 (s, 2 H) 5.81 (s, 2 H) 7.15 (s, 1 H) 7.89(d, J=8.00 Hz, 1 H) 7.98 – 8.05 (m, 1 H) 8.10 –8.18 (m, 1 H) 8.35 (dd, J=7.83, 1.96 Hz, 1 H) 8.39 (d, J=7.83 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com