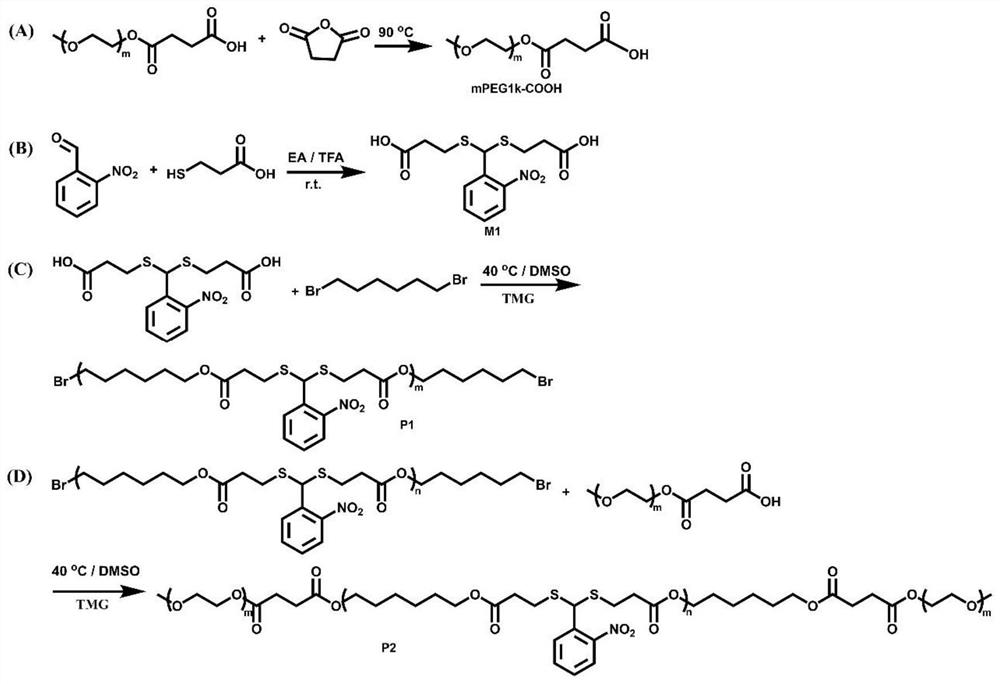
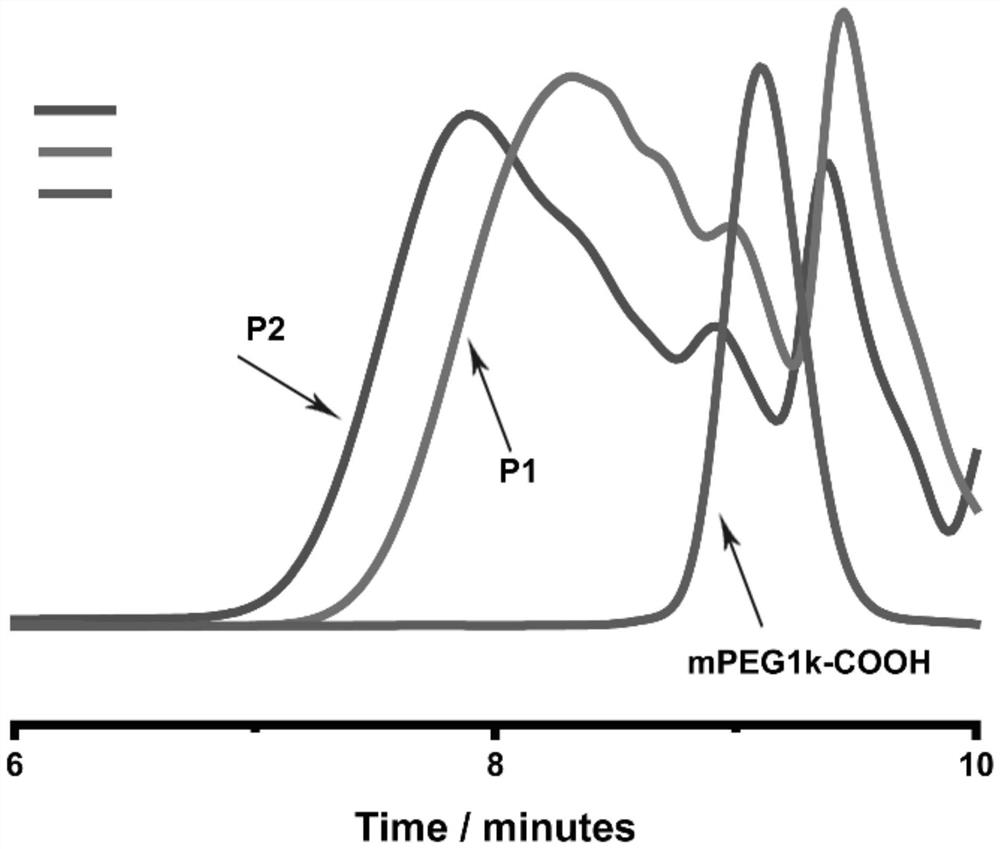
Degradable photoresponse polymer and preparation method thereof
A polymer and photoresponse technology, applied in the field of polymers, to achieve good photoresponse and degradation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of Polymer M1
[0039] 3-Mercaptopropionic acid (2.57 g, 22 mmol) and 2-nitrobenzaldehyde (1.51 g, 10 mmol) were dissolved in 25 mL of ethyl acetate, and 0.5 mL of trifluoroacetic acid was added to the mixture. The solution was stirred at room temperature for 24 hours. The solvent was then concentrated under reduced pressure. The crude product was further purified by column chromatography using EtOAc / petroleum alcohol (1 / 4, v / v) as eluent to give polymer M1.
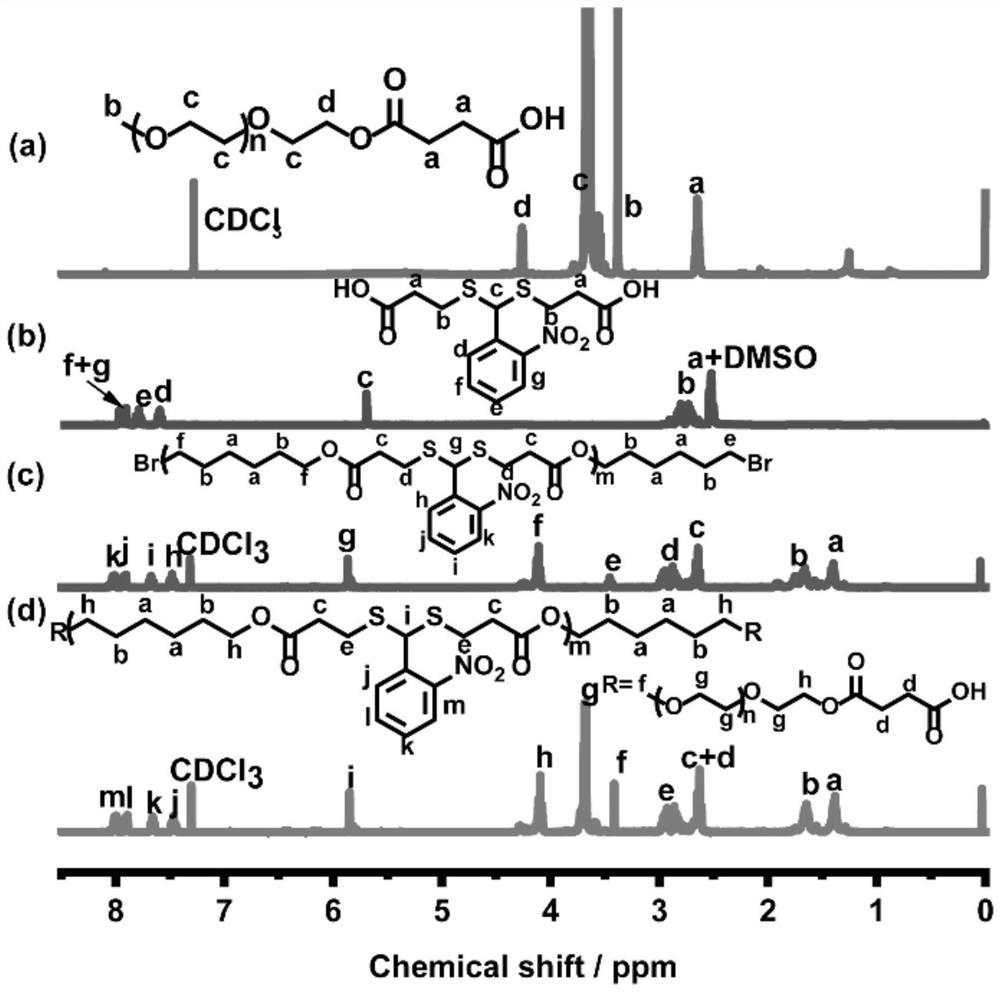
[0040] The polymer M1 obtained in Example 1 was subjected to nuclear magnetic test: refer to figure 2 a in
[0041] 1H NMR (400MHz, DMSO-d6) δ=7.96 (d, J=8.09, 1H), 7.94-7.88 (m, 1H), 7.83-7.75 (m, 1H), 7.63-7.55 (m, 1H), 5.69 (d, J=2.44, 1H), 2.97-2.62 (m, 4H), 2.58-2.41 (m, 4H).
Embodiment 2
[0043] Polyethylene glycol monomethyl ether mPEG-1K (5 g, 5 mmol) was mixed with succinic anhydride (0.55 g, 5.5 mmol). The mixture was stirred at 90°C for 18 hours. After that, the residual succinic anhydride was removed in vacuo at 110°C for 3 hours to obtain the polymer mPEG1k-COOH.
[0044] The polymer mPEG1k-COOH obtained in Example 2 was subjected to nuclear magnetic test: refer to figure 2 b in
[0045] 1H NMR (500mhz, CDCl3) δ 4.30-4.18 (m, 2H), 3.79-3.46 (m, 4H), 3.36 (s, 3H), 2.72-2.50 (m, 4H).
Embodiment 3
[0047] M1 (3.02 g, 8.75 mmol) and 1,6-dibromohexane (2.44 g, 10 mmol) were dissolved in DMSO (20 mL), followed by 1,1,3,3-tetramethylguanidine TMG (2.02 g) , 17.5 mmol) was slowly dropped into the mixture. The solution was stirred at 45°C for 12 hours to obtain polymer P1.
[0048] 2 ml of the polymer P1 solution obtained in Example 3 was poured into 10 ml of deionized water, and the precipitate was collected by filtration. The crude product was purified by dissolving in acetone, precipitating in diethyl ether and finally drying three times under vacuum at 45°C to obtain polymer P1 for NMR testing: reference figure 2 c in
[0049] 1HNMR (400MHz, CDCl3) δ 7.98 (dd, J=13.9, 7.8Hz, 1H), 7.86 (dd, J=8.2, 3.8Hz, 1H), 7.62 (t, J=7.6Hz, 1H), 7.42 ( t, J=7.8Hz, 1H), 5.86-5.73 (m, 1H), 4.29-3.96 (m, 4H), 3.40 (dd, J=9.0, 4.5Hz, 4H), 2.98-2.73 (m, 4H) , 2.73-2.54 (m, 77-1.54), 1.54 (m, 4H), 1.54-1.54 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com