Preparation method of reticulocyte quality control substance based on fluorescence principle
A technology of erythrocytes and erythrocytes, which is applied in the field of preparation of reticulocyte quality control substances based on the principle of fluorescence, and can solve the problems of increasing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
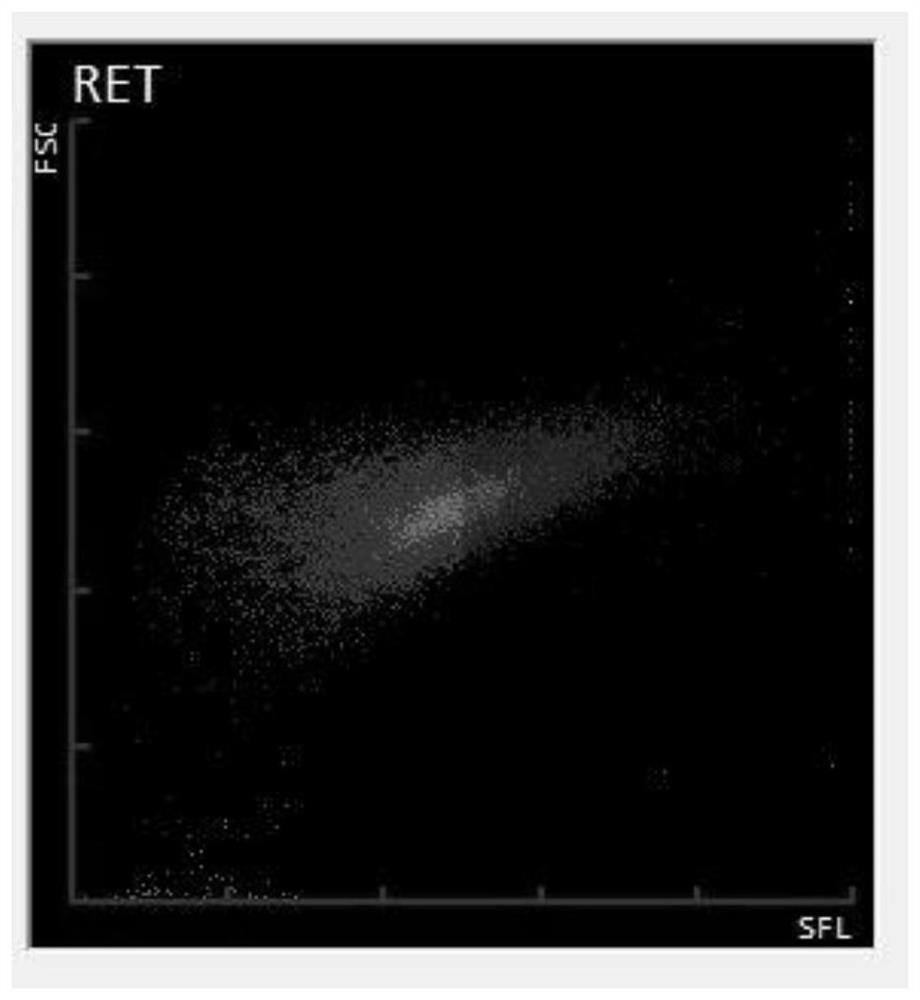
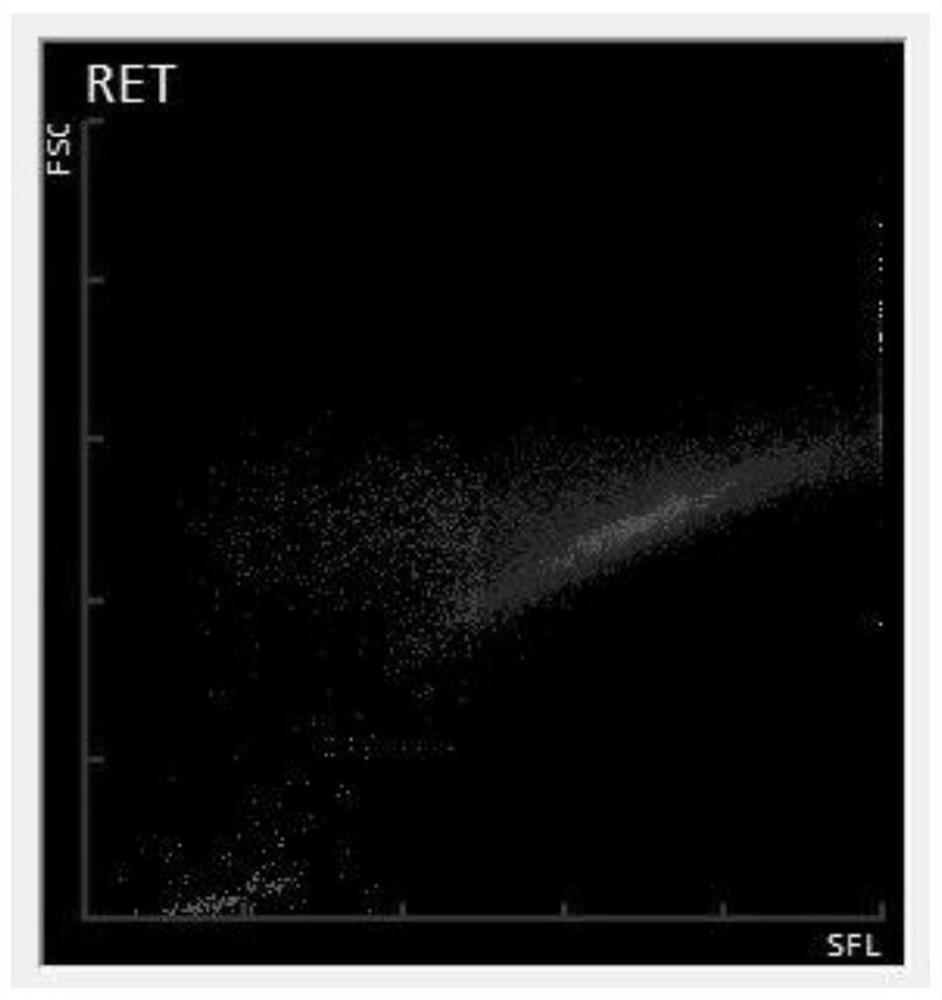
[0041] see Figure 1 to Figure 7 The present invention provides a method for preparing a reticulocyte quality control substance based on a fluorescence principle, comprising the following steps:
[0042] S1 washes and separates anticoagulated blood to obtain anucleated red blood cells;
[0043] The specific method is as follows: S11 mixes the anticoagulant with blood to obtain anticoagulated blood;
[0044] Specifically, the anticoagulant includes an EDTA anticoagulant or a citric acid anticoagulant;
[0045] S12 washes and separates the anticoagulated blood to obtain anucleated red blood cells.
[0046] S2 uses a cell solidifying agent to process and fix the anucleated red blood cells to obtain solidified red blood cells;
[0047] Specifically, the dosage of the cell fixative includes 5-20 times the volume of the anucleated red blood cells, and the greater the dosage of the cell fixative, the faster the reaction process.
[0048] The cell fixative includes aldehyde cell f...
Embodiment 1
[0068] Fresh anticoagulated pig blood was collected using anticoagulant. Pig blood was centrifuged at 2000 rpm for 10 minutes. The plasma, platelet layer and leukocyte layer are removed after centrifugation, leaving purified red blood cells. Add 10 times the volume of erythrocyte volume to the erythrocytes, and place it at room temperature for 40 minutes after mixing, and mix it every 10 minutes. After the treatment, centrifuge at 2000 rpm for 5 minutes, pour off the supernatant, add cell maintenance solution 3 times the volume of the remaining cells, add 5% of the total volume of the de-solidifying agent solution after mixing, and act at room temperature for 30 minutes. Repeat washing the cells two to three times with the cell maintenance solution, and then transfer the cells to an appropriate amount of cell maintenance solution for preservation, which can be used as a reticulocyte simulant. Red blood cell mimics, white blood cell mimics, platelet mimics, and nucleated red ...
Embodiment 2
[0070] Fresh anticoagulated pig blood was collected using anticoagulant. Pig blood was centrifuged at 2000 rpm for 10 minutes. The plasma, platelet layer and leukocyte layer are removed after centrifugation, leaving purified red blood cells. Add 10 times the volume of erythrocytes to the erythrocytes, and place the cell solidifying agent 2 at room temperature for 30 minutes after mixing, and mix once every 10 minutes. After the treatment, centrifuge at 2000 rpm for 5 minutes, pour off the supernatant, add cell maintenance solution 3 times the volume of the remaining cells, add 5% of the total volume of the de-solidifying agent solution after mixing, and act at room temperature for 30 minutes. Repeat washing the cells two to three times with the cell maintenance solution, and then transfer the cells to an appropriate amount of cell maintenance solution for preservation, which can be used as a reticulocyte simulant. Red blood cell mimics, white blood cell mimics, platelet mimi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com