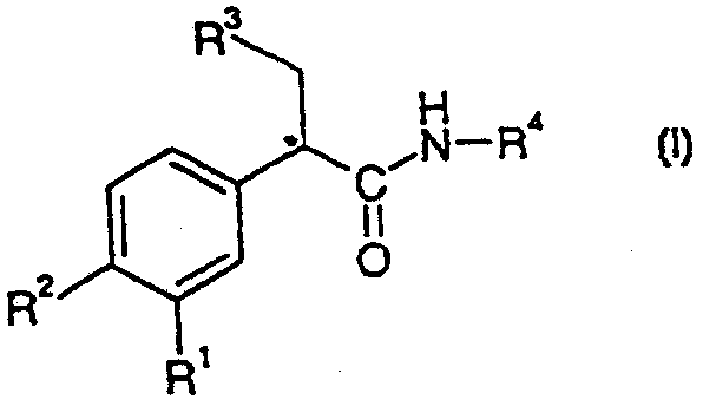
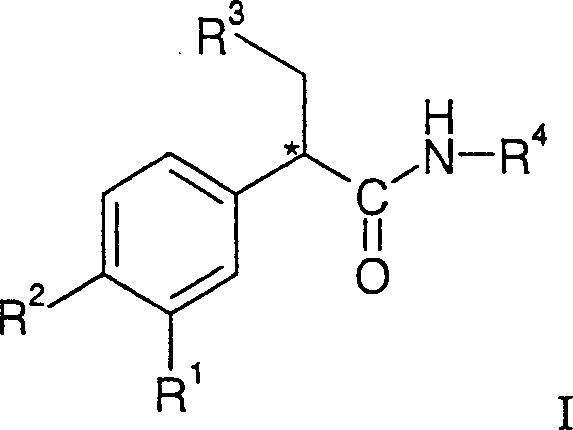
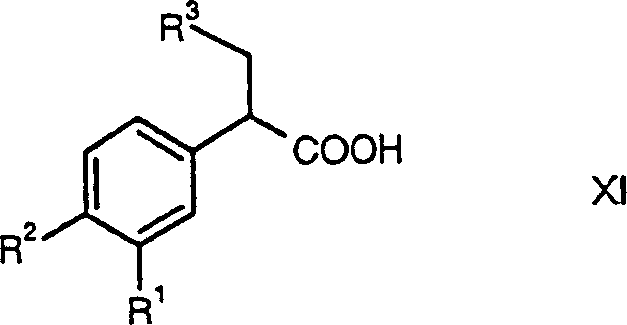
Glucokinase activators
A compound, low-level technology, applied in the direction of organic chemistry, organic active ingredients, chemical instruments and methods, etc., can solve problems such as increasing the sensitivity of GK sensor systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0445] (A) 3-cyclopentyl-2-(3,4-dichlorophenyl)-N-thiazol-2-yl-propionamide:
[0446]
[0447] 3-cyclopentyl-2-(3,4-dichlorophenyl)-propionic acid (prepared in Example 38, 2.0 g, 6.96 mmol), benzotriazol-1-yloxy-tris(dimethyl A solution of amino)phosphonium hexafluorophosphate (4.62g, 10.44mmol) and 2-aminothiazole (1.05g, 10.44mmol) in dichloromethane (50mL) was treated with triethylamine (2.9mL, 20.88mmol) deal with. The reaction mixture was stirred for 14h. The reaction mixture was then diluted with water (10 mL) and extracted with dichloromethane (3 x 10 mL). The combined organic layers were washed sequentially with water (1×10 mL), 1N aqueous sodium hydroxide solution (1×10 mL), 1N aqueous hydrochloric acid solution (1×10 mL) and saturated aqueous sodium chloride solution (1×10 mL). The organic layer was dried over sodium sulfate, filtered, and concentrated in vacuo. 3-Cyclopentyl-2-(3,4-dichlorophenyl)-N-thiazol-2-yl was obtained by flash chromatography (Merck Sil...
Embodiment 2
[0459] 2-(4-Bromo-phenyl)-3-cyclopentyl-N-thiazol-2-yl-propionamide
[0460]
[0461] Diisopropylamine (7.7mL, 54.88mmol) in dry tetrahydrofuran (23mL) and 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (10mL) The solution in was cooled to -78°C under nitrogen and then treated with a 2.5M solution of n-butyllithium in hexane (22.0 mL, 54.88 mmol). The resulting reaction mixture was stirred at -78 °C for 30 min, then added dropwise to dry THF (23 mL) and 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone ( The reaction mixture was treated with a solution of 4-bromophenylacetic acid (5.62 g, 26.13 mmol) in 10 mL). The color of the reaction mixture turned dark and was stirred at -78°C for 1 h while a solution of iodomethylcyclopentane (5.76 g, 27.44 mmol) in a small amount of dry THF was added dropwise. The reaction mixture was heated to 25 °C and stirred for 24 h. The reaction mixture was rapidly cooled with water, then concentrated in vacuo to remove tetrahydrofuran. ...
Embodiment 3
[0464] (A) 3-cyclopentyl-2-(4-methylsulfonyl-phenyl)-N-thiazol-2-yl-propionamide
[0465]
[0466]Diisopropylamine (3.3mL, 23.5mmol) in dry tetrahydrofuran (50mL) and 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (10mL) The solution was cooled to -78°C under nitrogen and then treated with a 10 M solution of n-butyllithium in hexane (2.35 mL, 23.5 mmol). The yellow reaction mixture was stirred at -78°C for 30 min, then a solution of 4-methylsulfonylphenylacetic acid (2.40 g, 11.2 mmol) in a small amount of dry THF was added dropwise. A solution of 4-methylsulfonylphenylacetic acid in dry tetrahydrofuran was added about halfway, and a precipitate formed. Addition of the remaining solution of 4-methylsulfonylphenylacetic acid in dry THF was continued and the reaction mixture thickened substantially. After complete addition of the solution of 4-methylsulfonylphenylacetic acid in dry THF, the reaction mixture became very viscous and difficult to stir. Additional dry THF ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com