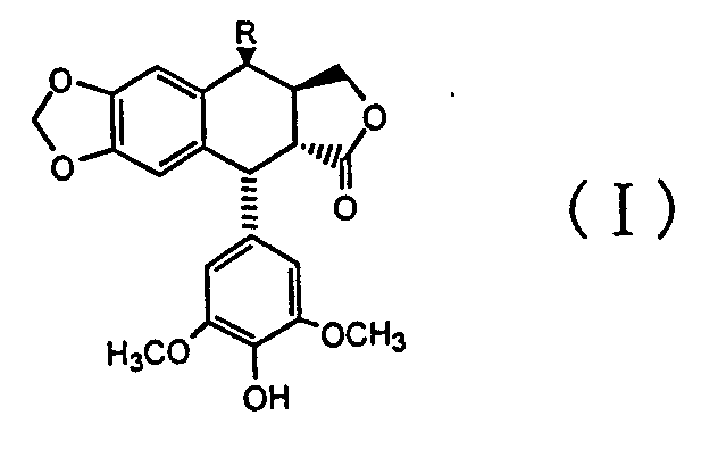
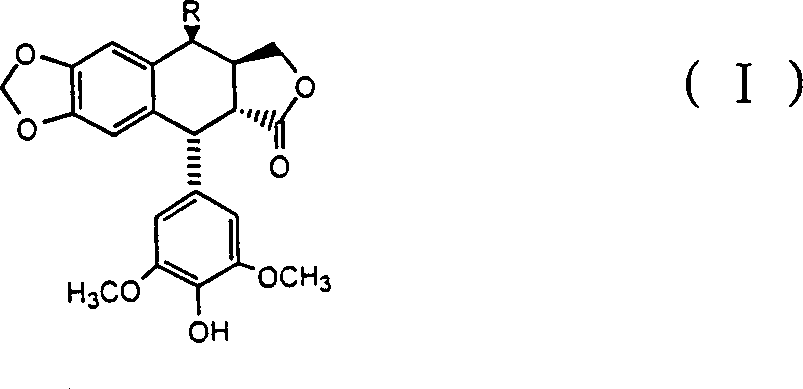
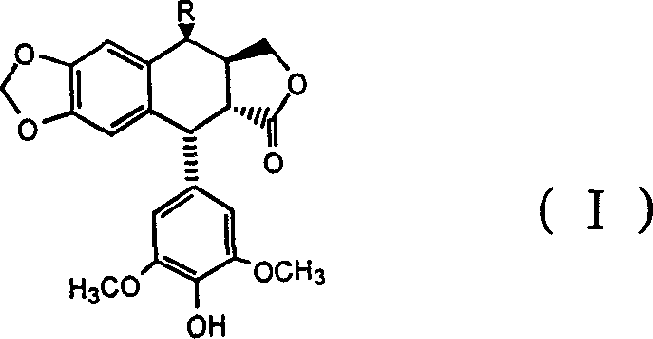
4 beta-benseleno-4-desoxy-4-demethylpodophyllotoxin derivant
A nitrobenzyl selenium-based and selenium-based technology, which is applied in the field of compound preparation of anti-tumor drugs, can solve the problems of myelosuppression, high toxicity, and clinical application limitations, and achieve the effect of improving anti-tumor activity and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of 4β-benzylselenyl-4-deoxy-4′-desmethylpodophyllotoxin
[0015] Add 4α-podophyllotoxin (II), hydrogen bromide and solvent dichloromethane into the reaction vessel to obtain the product 4β-bromo-4-deoxy-4′-demethylpodophyllotoxin ( III), then benzyl diselenide, sodium borohydride, ethanol and tetrahydrofuran are added, and the compound of the present invention, namely 4β-benzylselenyl-4-deoxy-4′-desmethylpodophyllotoxin (I ) (The substituent R in the formula is benzylselenyl), and its melting point is 129-130°C.
[0016] The relevant detection data of the obtained product of the above structural formula (I) are as follows:
[0017] Mass spectrum (FAB-MS): relative kurtosis (%) m / z
[0018] (1) 100 383
[0019] (2) 6 341
[0020] (3) 25 299
[0021] (4) 6 267
[0022] (5) 31 229
[0023] IR(KBr, cm -1 ): 3440 (OH), 1750 (γ-lactone, C=O), 1610, 1510, 1500 (benzene ring, C=C), 1238, 1190 (C-O-C), 930 ...
Embodiment 2
[0026] Preparation of 4β-p-nitrobenzylselenyl-4-deoxy-4′-desmethylpodophyllotoxin
[0027] Add 4α-podophyllotoxin (II), hydrogen bromide and solvent dichloromethane into the reaction vessel to obtain the product 4β-bromo-4-deoxy-4′-demethylpodophyllotoxin ( III), then add p-nitrobenzyl diselenide, sodium borohydride, ethanol and tetrahydrofuran, and obtain the compound of the present invention, namely 4β-p-nitrobenzylselenyl-4-deoxy-4'-nor Podophyllotoxin (I) (the substituent R in the formula is p-nitrobenzylselenyl), and its melting point is 166-168°C.
[0028] The relevant detection data of the obtained product of the above structural formula (I) are as follows:
[0029] IR(KBr, cm -1 ): 3500 (OH), 1755 (γ-lactone, C=O), 1620, 1510, 1480 (benzene ring, C=C), 1540, 1335 (NO 2 ), 1285, 1150(C-O-C), 935(OCH 2 O).
[0030] 1 H-NMR(CDCL 3 , TMS500Mz)δppm: 7.93(d, 2H, 3″, 5″-H), 7.51(d, 2H, 2″, 6″-H), 6.66(s, 1H, 5-H), 6.57(s, 1H, 4-H), 6.34 (s, 2H, 2', 6'-H), 5.95, 5.91 (brs, J = 1.2...
Embodiment 3
[0032] 4β-p-fluorobenzselenyl-4-deoxy-4′-desmethylpodophyllotoxin
[0033] Add 4α-podophyllotoxin (II), hydrogen bromide and solvent dichloromethane into the reaction vessel to obtain the product 4β-bromo-4-deoxy-4′-demethylpodophyllotoxin ( III), then add p-fluorobenzyl diselenide, sodium borohydride, ethanol and tetrahydrofuran, and obtain the compound of the present invention, namely 4β-p-fluorobenzylselenyl-4-deoxy-4′-demethylated Phlotoxin (I) (the substituent R in the formula is p-fluorobenzylselenyl).
[0034] The relevant detection data of the obtained product of the above structural formula (I) are as follows:
[0035] IR(KBr, cm -1 ): 3500 (OH), 1755 (γ-lactone, C=O), 1620, 1510, 1480 (benzene ring, C=C), 1285, 1150 (C-O-C), 1050 (C-F), 935 (OCH 2 O).
[0036] 1 H-NMR(CDCL 3 , TMS500Mz)δppm: 7.28, 7.06, 7.01, 6.95 (each m, 1H, 2″, 4″, 5″, 6″-H), 6.50(s, 1H, 5-H), 6.39(s, 2H, 2', 6'-H), 6.01, 5.97 (brs, J=1.2, 7.5Hz, 2H, -OCH 2 O-), 5.43 (s, 1H, -OH), 4.47 (d, 1H, 4-H), 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com