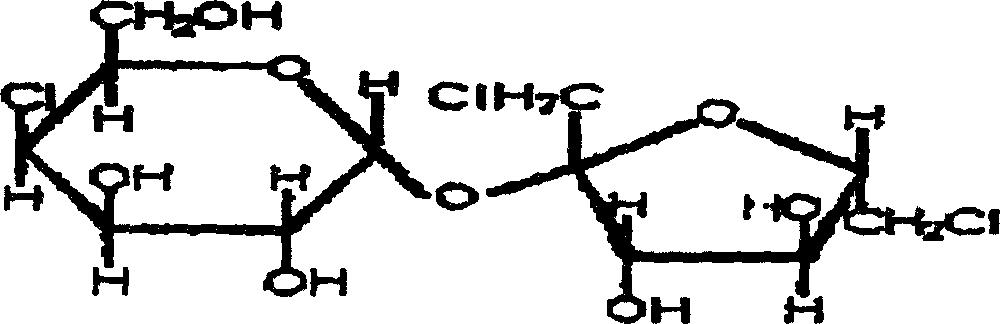
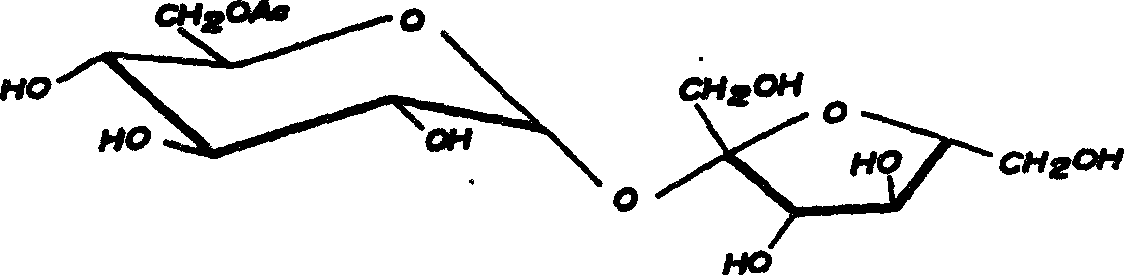Synthesis of sucrose-6-acetate
A synthesis method and acetate technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of complex process and high production cost, and achieve simple process, low production cost and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of supported cerium sulfate-silica gel catalyst
[0015] Add 6.2g Ce(SO 4 ) 2 4H 2 O, add 20ml of water to dissolve, then add 4.8g of 230-400 mesh silica gel, heat to 110°C on a rotary evaporator and dry under vacuum (200Pa) for 3 hours to obtain about 9.5 grams of cerium sulfate-silica gel (1:1) catalyst.
Embodiment 2
[0017] In a 1000ml three-necked flask, add 50g of sucrose, 250ml of N,N-dimethylformamide (DMF), 2g of anhydrous cerium sulfate and 50ml of ethyl acetate, heat to 80°C and stir for 4 hours, then cool to 10°C , filter and recover cerium sulfate, first distill off the unreacted ethyl acetate at room temperature, and then distill all the DMF under reduced pressure. Obtain 55g of syrup, detect by HPLC, the content of sucrose-6-acetate is 79.3%.
Embodiment 3
[0019] In a 1000ml three-necked flask, add 50g of sucrose, 250ml of N,N-dimethylformamide (DMF), 5g of cerium sulfate-silica gel (1:1), 100ml of ethyl acetate, heat to 80°C and stir for 4 hours , cooled to 5°C, and the catalyst was recovered by filtration. First, the unreacted ethyl acetate was evaporated at room temperature, and then all the DMF was evaporated under reduced pressure to obtain 56 g of syrup. The content of sucrose-6-acetate was 85.7% by HPLC detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com