Thermosensitive cyclotriphosphazene-platinum complex conjugate, its preparation method and anticancer agent containing the same
A technology of cyclotriphosphazene and conjugates, which is applied in the field of cyclotriphosphazene-platinum composite conjugates, and can solve the problems of high price, low anticancer activity and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
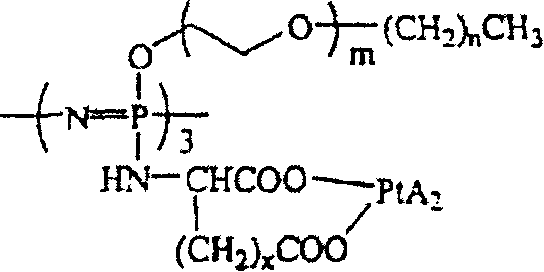
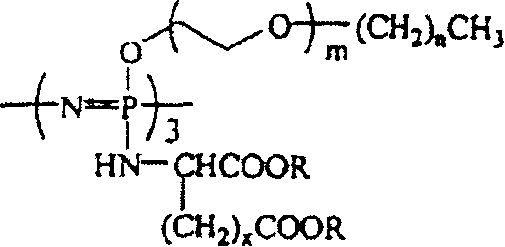
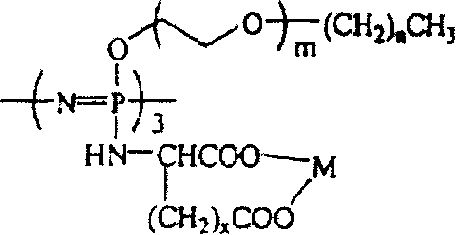
[0031] The preparation method of the present invention can be represented graphically by the following reaction scheme 1:
[0032] Reaction scheme 1
[0033]
Embodiment 1
[0038] {NP[(OCH 2 CH 2 ) 2 OCH 3 ][L-Asp·Pt(dmpda)] 3 preparation of
[0039] Will {NP[(OCH 2 CH 2 ) 2 OCH 3 ][NHCH(CH 2 COOC 2 h 5 )COOC 2h 5 ]} 3 (1.00g, 0.95mmol) was dissolved in methanol (50ml), and the temperature of the reaction vessel was controlled at 0-5°C with an ice-water bath. Slowly add the solution containing excess Ba(OH) under stirring 2 ·8H 2 O (1.19 g, 3.78 mmol) in methanol. The ice-water bath was removed after 30 minutes, and the reaction was continued for 3 hours. The reaction mixture was concentrated under reduced pressure until only a small amount of solvent remained. Excess ether or hexane was added to the concentrated reaction mixture to cause precipitation of the hydrolyzate. The precipitate thus obtained is redissolved in a small amount of methanol and then reprecipitated by adding excess ether or hexane. After the precipitation process was repeated 2-3 times, the final precipitate was vacuum-dried to obtain 0.99 g of cyclotripho...
Embodiment 2
[0053] {NP[(OCH 2 CH 2 ) 2 OCH 3 ][L-Asp·Pt(dach)]} 3 preparation of
[0054] Use {NP[(OCH 2 CH 2 ) 2 OCH 3 ][NHCH(CH 2 COOC 2 h 5 )COOC 2 h 5 ]} 3 (0.73g, 0.69mmol), Ba(OH) 2 ·8H 2 O (0.87g, 2.76mmol), cyclotriphosphazene-barium salt (0.69g, 0.61mmol), and (dach)PtSO 4 (0.63g, 1.83mmol), obtain the final conjugated product {NP[(OCH 2 CH 2 ) 2 OCH 3 ][L-Asp·Pt(dach)]} 3 (1.05 g, 95.5% yield).
[0055] Molecular formula: C 45 h 87 N 12 o 21 P 3 Pt 3
[0056] Elemental analysis (%): C, 29.01; H, 4.79; N, 9.39; P, 5.01; Pt, 30.95
[0057] Theoretical values: C, 29.85; H, 4.84; N, 9.28; P, 5.13; Pt, 32.33
[0058] Proton NMR spectroscopy (D 2 O, ppm):
[0059] δ1.1-1.4(b, 4H,
[0060] δ1.5.1.7(b, 2H,
[0061] δ2.0-2.2(b, 2H,
[0062] δ2.3-2.5(b, 2H,
[0063] Phosphorus NMR spectroscopy (D 2 O, ppm): δ34-44
[0064] Lower critical solution temperature: 92.0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com