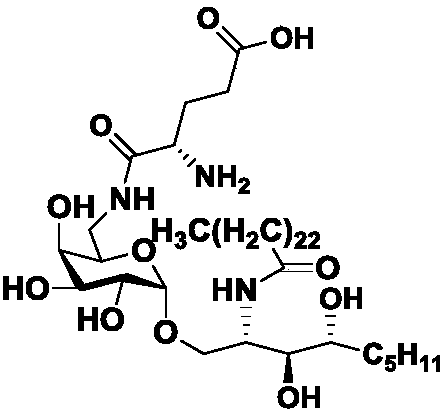
Compound capable of selectively inducing Th2 immune cytokine production and potential application thereof
A technology of immune cells and compounds, applied in the fields of chemistry and medicine, can solve problems affecting Th1/Th2 selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
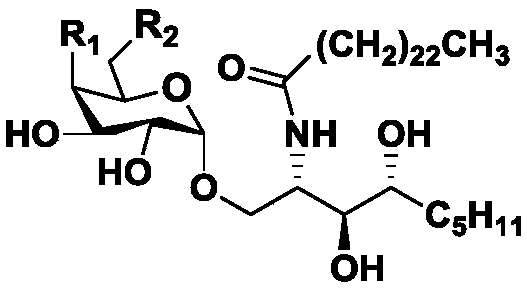
[0028]
[0029] The following provides embodiments of the present invention (taking the above HU_ZHAO_2018-1 as an example):
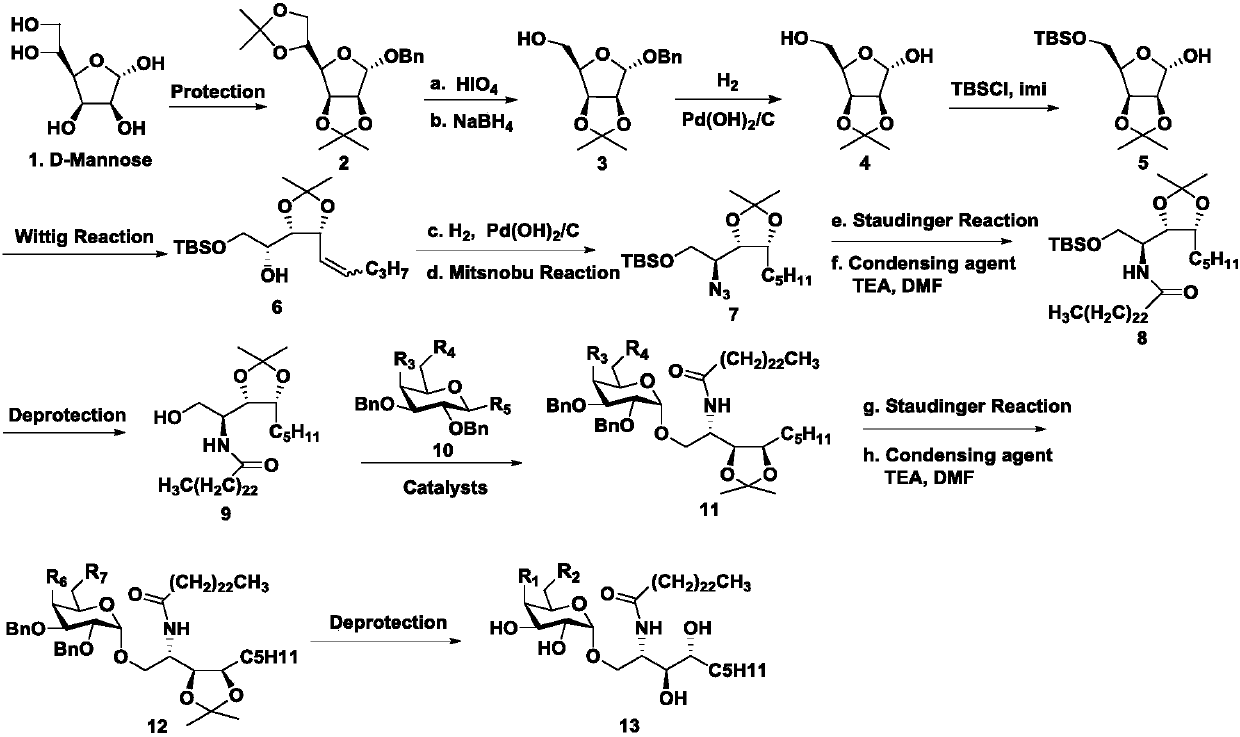
[0030] Synthesis of compound 2:
[0031] At room temperature, mannose (1 eqv) was dissolved in acetone solution, a catalytic amount of iodine was added, the reaction was carried out at room temperature for 5 h, quenched with saturated sodium thiosulfate solution, evaporated to dryness, extracted with ethyl acetate and water, the organic phase was dried, and concentrated The crude product was obtained, the crude product was dissolved in tetrahydrofuran solution, solid potassium hydroxide (1.8eqv), catalytic amount of 18-crown-6, benzyl bromide (1.1eqv) were added successively, the reaction was carried out at room temperature for 30min, and saturated ammonium chloride solution was added. The reaction was quenched, extracted with ethyl acetate and water, the organic phase was dried, concentrated, and column chromatography gave Intermediate 2 in 80% ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com