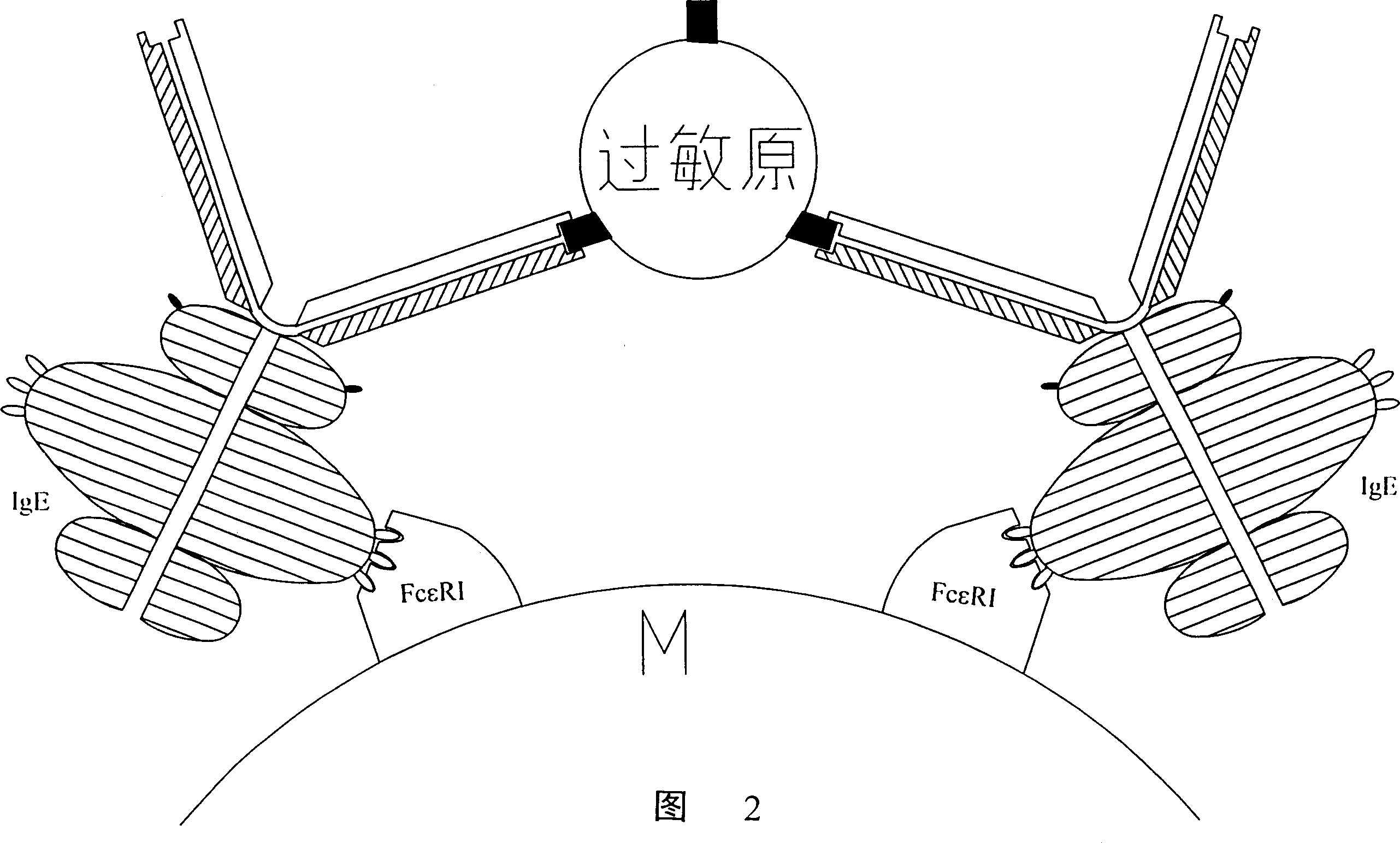
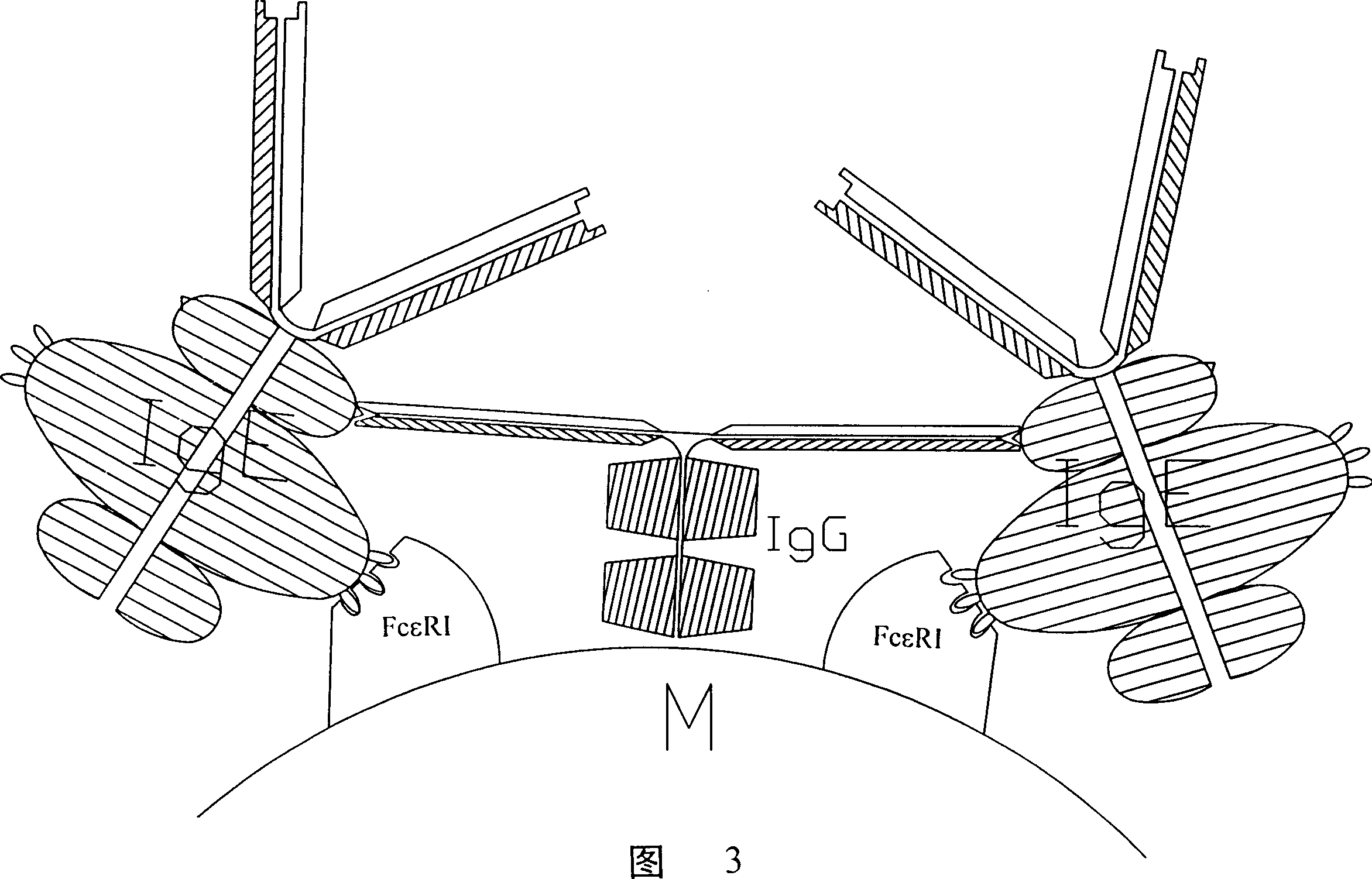
Peptide immunogen, vaccine made thereof for curing allergic reaction and its preparation method
An immunogen and immunogenic technology, applied in the field of molecular immunology, can solve the problems of insufficient use and the effect of population vaccination remains to be seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The chemical synthesis of embodiment 1 human IgE polypeptide and the preparation of liposome vaccine
[0102] (1) Chemical synthesis of peptides
[0103]According to the amino acid residue sequences of the five groups of polypeptides designed in the present invention, A, B, C, D, and E, respectively, on the ABI 431 automatic polypeptide synthesizer (Applied Biosystemes company), according to the operating instructions, carry out solid-phase chemistry one by one. Synthesis (Sheppard RC: Science Tools 1986, 33:9-16). The HMP resin, F-moc series of activated amino acids and main chemical reagents used are all products of ABI Company. After the synthesis is completed, the dry peptide-resin is taken out, and a pre-cooled dissociation mixture (0.75 g of crystalline phenol, di Thioethylene ether 0.25ml, thioanisole 0.5ml, deionized water 0.5ml, trichloroacetic acid 10ml), the amount added is based on 0.1-1.5g peptide resin plus 10ml. The reaction was stirred at room temperat...
Embodiment 2
[0110] Except using KLH (product of Piere, USA) as the carrier protein, the polypeptide-carrier protein was prepared according to the method of Example 1 (2) and (3) and liposome vaccine was further prepared.
Embodiment 3
[0112] Purified TT (semi-finished product of corresponding vaccine) without protective agent was used as carrier protein. Take 2 mg of this carrier protein to prepare a solution: take 2 mg of A-4 polypeptide and C-1 polypeptide (or A-1 polypeptide and E-1 polypeptide, etc.) and dissolve in PBS. After the carrier protein solution was mixed with the mixed polypeptide solution, glutaraldehyde solution was added to make the final concentration 0.2%, and the dipeptide-carrier protein complex was obtained in the same manner as in Example 1 (two), and used in the same manner as in Example 1 ( 3) Vaccines are prepared in the same way.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com