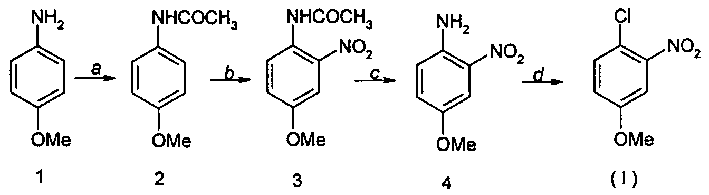
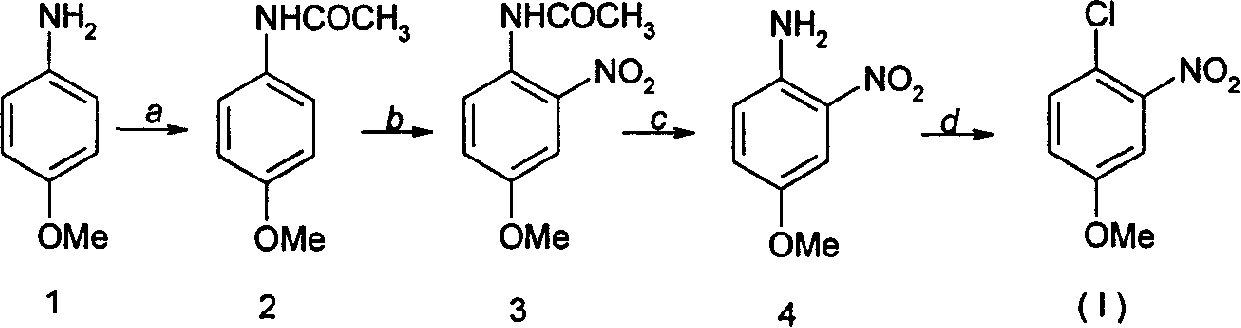
Method for preparing 4-chlorine-3-nitro methyl-phenoxide
A technology for nitroanisole and p-aminoanisole is applied in the field of synthesizing 4-chloro-3-nitroanisole, and can solve problems such as difficulty in industrialized production, high cost, and difficulty in popularizing chemical tests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] 1, the preparation of p-methoxyacetanilide (compound 2)
[0015] Add 13.1kg of acetic anhydride into a 20L reaction kettle, add 7.38kg of compound 1 (p-aminoanisole) under stirring, add 27.0g of p-aminoanisole and 32.4ml of water into a 250ml reaction bottle, and stir. Add 21.6ml of acetic anhydride, stir to form a solution, cool to room temperature, precipitate loose solids, filter a large amount of oil into the filtrate, wash with water to obtain a crude product, recrystallize with water, remove a small amount of oil with activated carbon, and obtain colorless flaky crystals The product was dried to obtain 20.5 g of p-methoxyacetanilide, yield: 56.6%, mp: 127°C.
[0016] 2, Preparation of 4-amino-3-nitroanisole (compound 4)
[0017] In a 50L reactor, add 7.8kg of p-methoxyacetanilide, 55g of sodium nitrate dissolved in 120ml of water, 180g of fuming nitric acid dissolved in 30kg of dichloromethane solution, after heating to reflux, stop heating, dropwise add 3.44kg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com