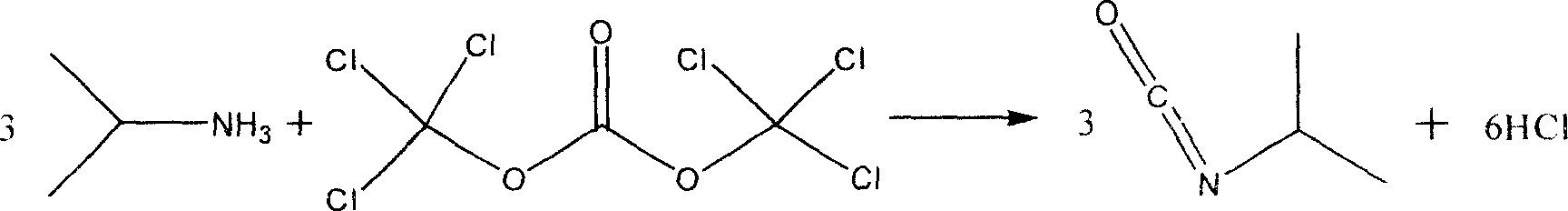
Chemical synthesis method of isopropyl isocyanate
A chemical synthesis technology of isopropyl isocyanate, applied in chemical instruments and methods, preparation of isocyanic acid derivatives, organic chemistry, etc., can solve potential safety hazards, difficulties in storage and transportation, loss of human life, property and environment, etc. problems, to achieve the effect of no three wastes, low production cost, great implementation value and social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] The feed molar ratio is isopropylamine: bis(trichloromethyl) carbonate: N-methylpyrrole=1:0.35:0.1.
[0009] In a 500ml four-necked flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add 1mol isopropylamine and 200ml solution of tetrahydrofuran, turn on the stirring, slowly add dropwise to 100ml tetrahydrofuran under vigorous stirring at room temperature The bis(trichloromethyl)carbonate solution of bis(trichloromethyl)carbonate is added, heated to reflux, and reacted at 60-68℃ for 3h. After the reaction is completed, nitrogen gas is used to drive off the hydrogen chloride gas. The tetrahydrofuran is recovered by vacuum distillation and the product 42.5g is distilled out. The rate is 50.3%, and the content is 99.6% (GC).
Embodiment 2
[0011] The feed molar ratio is isopropylamine: bis(trichloromethyl) carbonate: N-methylpyrrole=1:0.45:0.1.
[0012] In a 500ml four-necked flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add 1mol of isopropylamine and 180ml of tetrahydrofuran toluene, turn on the stirring, and slowly add dropwise dissolved in 120ml of toluene under vigorous stirring at room temperature. The bis(trichloromethyl)carbonate solution of bis(trichloromethyl)carbonate is added, the temperature is raised to reflux, and the reaction is stirred at 70-75°C for 4h. After the reaction is completed, nitrogen is used to drive off the hydrogen chloride gas, and 45.1g of the product is evaporated by vacuum distillation. The rate is 53%, and the content is 99.5% (GC).
Embodiment 3
[0014] The feed molar ratio is isopropylamine: bis(trichloromethyl) carbonate: N-methylpyrrole=1:1.5:0.1.
[0015] In a 500ml four-necked flask equipped with mechanical stirring, constant pressure dropping funnel, reflux condenser and thermometer, add 1mol isopropylamine and dichlorobenzene 180ml solution, turn on the stirring, and slowly add the dissolved solution at room temperature under vigorous stirring. 120ml of toluene bis(trichloromethyl)carbonate solution, after the addition, heat to reflux, and stir the reaction at 70-80℃ for 3h. After the reaction is completed, nitrogen gas is used to drive off the hydrogen chloride gas, and the product 46.8g is evaporated by vacuum distillation The yield is 55%, and the content is 99.8% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com