Rhodiola sacra glycoside derivative and its preparation method and use
A technology of salidroside and derivatives, applied in the field of medicine, can solve the problems of cumbersome reaction operation, low total yield and high cost, and achieve the effects of simplifying the process, reducing pollution and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
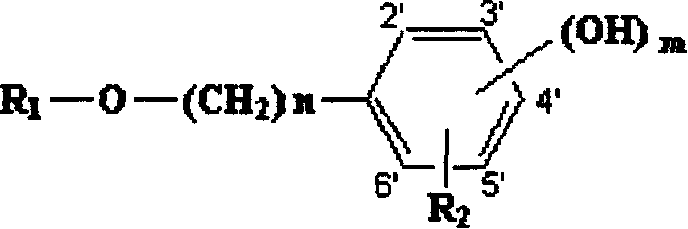
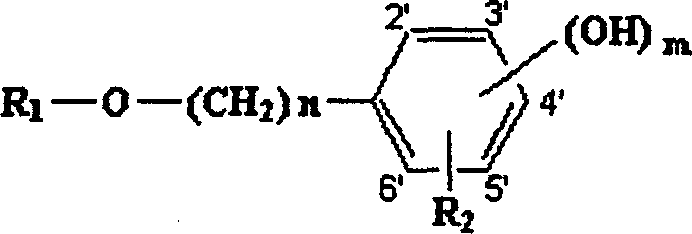
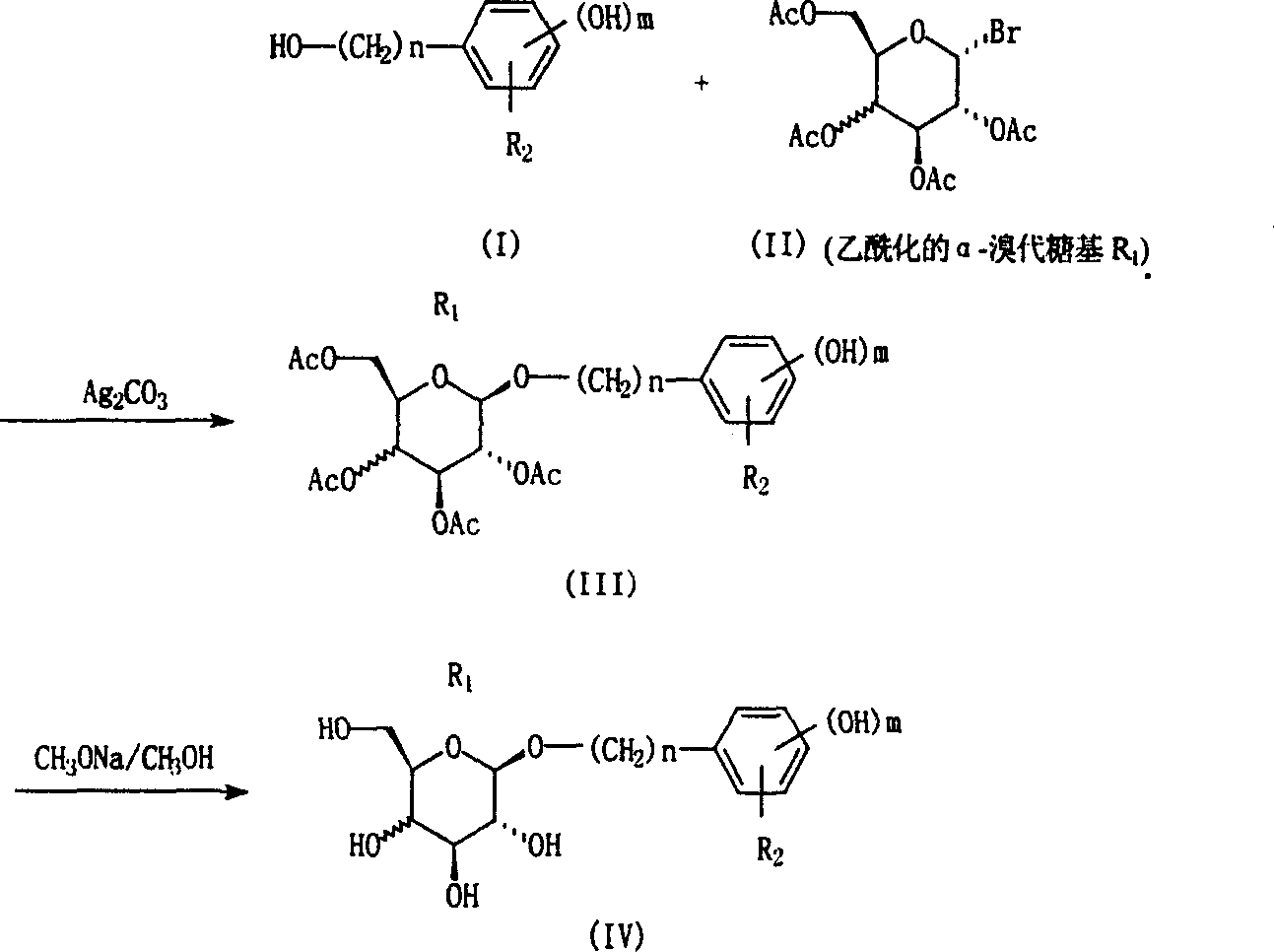
[0041] Example 1: Preparation of 1-(4-hydroxy)phenethyl-β-D-glucopyranoside (salidroside, Compound No. 1).
[0042] Nitrogen was passed into a 50ml round bottom flask for protection, 30ml of anhydrous dichloromethane, 1g (7.5mmol) of 4-hydroxyphenethyl alcohol, 1.65g (6mmol) of silver carbonate were added, and the reaction was carried out at room temperature for 30 minutes in the dark under stirring . Add 2.65 g (6.5 mmol) of 2,3,4,6-tetra-O-acetyl-α-D-bromoglucopyranose and 0.83 g (3 mmol) of silver carbonate, and react at room temperature for 24 hours. After filtration, the solvent was distilled off under reduced pressure to obtain a viscous colorless residue, which is 1-(4-hydroxy)phenylethyl-β-D-glucopyranoside acetylated with sugar hydroxy groups.
[0043] Add 20ml (27mM) of methanol solution dissolved with sodium methoxide to the above residue, react at room temperature for 20 hours, concentrate under reduced pressure, separate with silica gel G column chromatography, a...
Embodiment 2
[0044] Example 2: Preparation of 1-(4-hydroxy)phenethyl-β-D-galactopyranoside (Compound No. 17).
[0045] Nitrogen was passed into a 50ml round bottom flask, 30ml of anhydrous dichloromethane, 1g (7.5mmol) of 4-hydroxyphenethyl alcohol, 1.65g (6mmol) of silver carbonate were added, and the reaction was carried out at room temperature in the dark for 30 minutes under stirring, and then added 2.65 g (6.5 mmol) of 2,3,4,6-tetra-O-acetyl-α-D-bromogalactopyranose and 0.83 g (3 mmol) of silver carbonate were reacted at room temperature for 24 hours. After filtration, the solvent was distilled off under reduced pressure to obtain a viscous colorless liquid, which was 1-(4-hydroxy)phenethyl-β-D-galactopyranoside acetylated with sugar hydroxy groups.
[0046] Add 20ml (27mM) of methanol solution dissolved with sodium methoxide into the above solution, react at room temperature for 20 hours, concentrate under reduced pressure, separate with silica gel G column chromatography, and use ch...
Embodiment 3
[0047] Example 3: Preparation of 1-(3-hydroxy)phenethyl-β-D-glucopyranoside (Compound No. 2).
[0048] Nitrogen was passed into a 50ml round bottom flask, 30ml of anhydrous dichloromethane, 1g (7.5mmol) of 3-hydroxyphenethyl alcohol, 1.65g (6mmol) of silver carbonate were added, and the reaction was carried out at room temperature in the dark for 30 minutes under stirring, and then added 2,3,4,6-Tetra-O-acetyl-α-D-bromoglucopyranose 2.65g (6.5mmol), silver carbonate 0.83g (3mmol), react at room temperature for 24 hours, filter, evaporate under reduced pressure Solvent, a viscous colorless residue was obtained, which was sugar hydroxyacetylated 1-(3-hydroxy)phenethyl-β-D-glucopyranoside.
[0049] Add 20ml (27mM) of methanol solution dissolved with sodium methoxide to the above residue, react at room temperature for 20 hours, concentrate under reduced pressure, separate with silica gel G column chromatography, use chloroform and methanol (4:1) as developing solvent, and obtain t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com